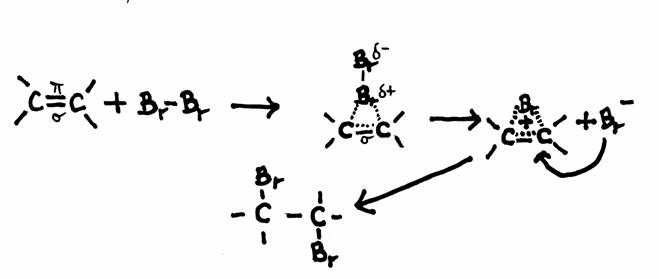
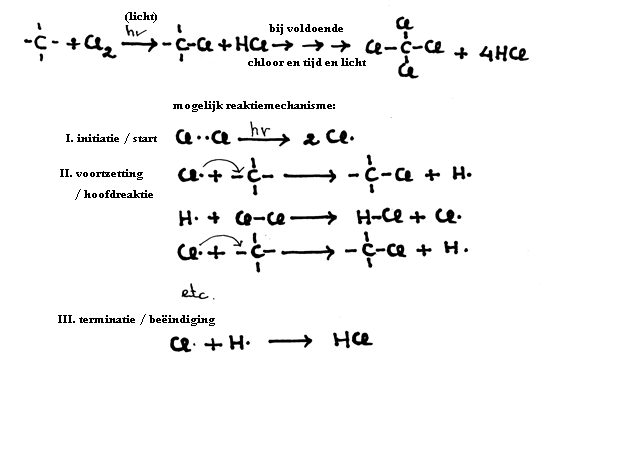
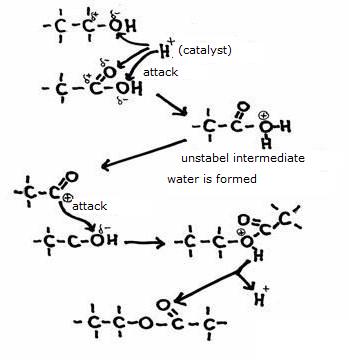
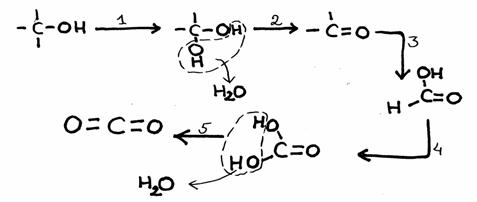
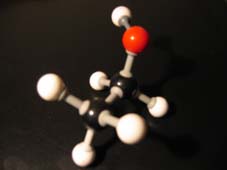
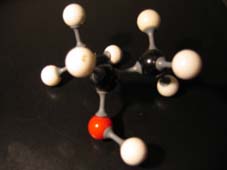
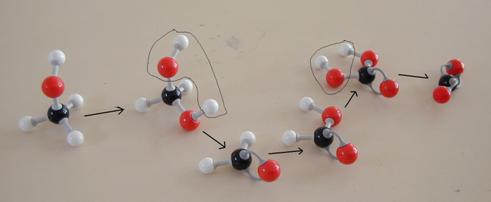
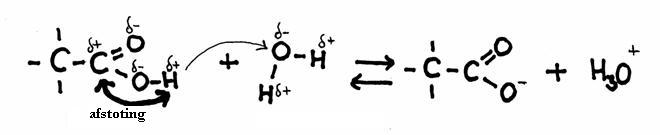
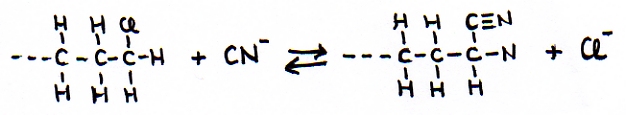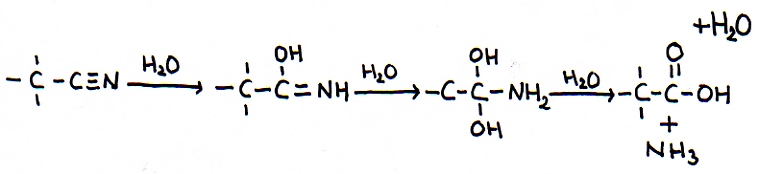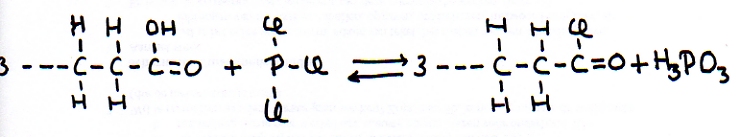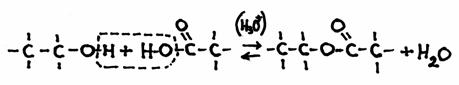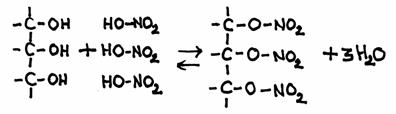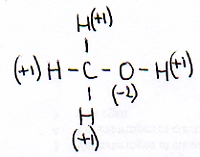The other open side still remains open, and is ready to continue the attack on another molecule of the same type:
This part of the process is called: initiation:
Cl – Cl
Cl· + CH2 = CH – CH3
This radical attacks the molecules that are present in good amounts: the other molecules of propene:
-
C3H6Cl· + C3H6
 C6H12·
C6H12·
-
C6H12· + C3H6
 C9H18·
C9H18·
-
etcetera
This is a chain reaction causing enormous chains. Every time a bigger radical is made that every time again can catch a monomere. In this way macromolecules are formed.
Those two electrons immediately form a normal covalent bond; the action is over and the polymerisation comes to an end.
There are different ways to close such a chain reaction:
Termination: CnH2n· + Cl·
or:
CnH2n · + CnH2n·