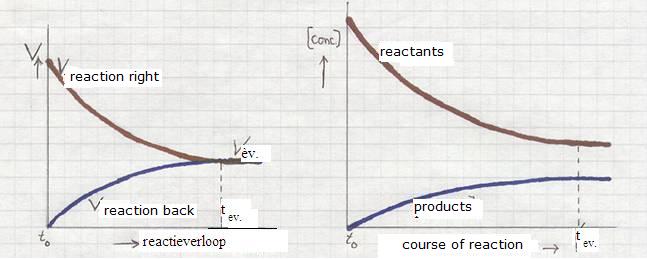
How will change the reaction rates of the forward and the backward reaction between start (t0) and finish (the equilibrium is reached (teq)?
The stronger substances react in favor of the weaker ones |

| Concentration of saccharose |
Concentration of glucose |
Concentration of fructose |
Duration of the reaction in minutes |
|---|---|---|---|
1.00 |
1.00 |
0 |
|
0.20 |
0.79 |
60 |
|
0.67 |
120 |
||
0.40 |
0.60 |
180 |
|
240 |
|||
0.50 |
300 |
 In the beaker is NH3(aq) (basic environment) with some drops of the indicator methyl red,
In the beaker is NH3(aq) (basic environment) with some drops of the indicator methyl red,
 Now you add a bit of HCl(aq), the basic environment changes to a less basic or more aced env.
Now you add a bit of HCl(aq), the basic environment changes to a less basic or more aced env.
 Now again we add some base:
Nu gaan we weer base toevoegen: (NH3(aq)), the color changes again to yellow
Now again we add some base:
Nu gaan we weer base toevoegen: (NH3(aq)), the color changes again to yellow
 Doing this can be repeated endless.
Doing this can be repeated endless.
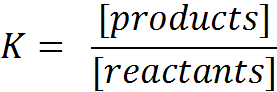
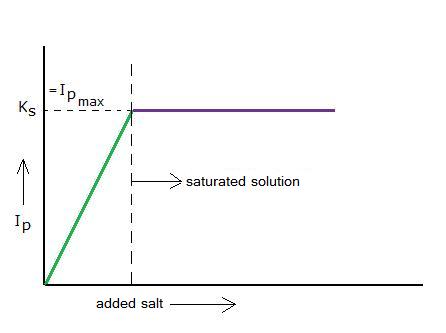
| H2O | + | H2O | H3O+ | + | OH- | ΔH | > 0 | ||
|
|
|
|
|
|
|
|
| |