|
This is a module about atoms, where you can learn how atoms are constructed.
It is on a basic level, but yet you wil understand more about atoms, their discovery history, how they are composed, about their important properties. Besides that, concepts like 'ion', 'atom mass', 'electron formula' and radio activity' wil come up. |
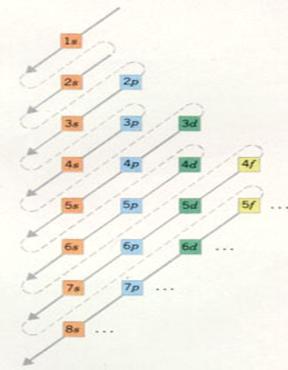
The further from the nucleus, the more energy has an electron.
The own energy of an electron
that is under the influence of the powers of an atom nucleus,
determines in what energy level that electron may stay.
| Main level 1 has only one sublevel, |
of the type s | with a maximum electron number of: |
2 |
| Main level 2 has 2 sublevels |
of the type s and p | with a maximum electron number of: |
2 and 6 |
| Main Level 3 has 3 sublevels |
of the type s, p and d | with a maximum electron number of: |
2, 6 and 10 |
| Main level 4 has 4 sublevels |
of the type s, p, d and f | with a maximum electron number of: |
2, 6 , 10 and 14 |
|
The main levels 5 to 7 - theoretically - could be divised in 5, 6 and 7 sublevels.
But no such big atoms exist; nature did not realise them. so: |
|||
| Main level 5 divides itself in 4 sublevels |
of the type s, p, d and f | with a maximum electron number of: |
2, 6, 10 and 14 |
| Main level 6 divides itself in 3 sublevels |
of the type s, p and d | with a maximum electron number of: |
2, 6 and 10 |
| Main level 7 divides itself in two sublevels |
of the type s and p | with a maximum electron number of: |
2 and 6 |
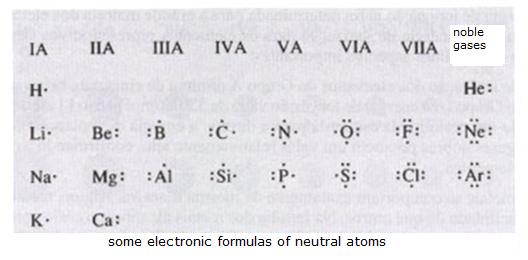
| Particle | Mass (g) | Mass (a.m.u) | Charge |
| Electron | 9,1*10-28 | 0 | -1 |
| Neutron | 1,67495*10-24 | 1 | 0 |
| Proton | 1,67254*10-24 | 1 | +1 |
| Sodium | yellow | Copper | green | |
| Potassium | weak violet | Stannium | blue | |
| Calcium | red | Lead | weak blue |