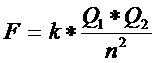| The chemical element is a substance that we cannot - by chemical means - split into other substances. |
| Every element has its own atom number and its own symbol |
| Every symbol is a CAPITAL, sometimes with a small letter |
|
3. The elements
|
|
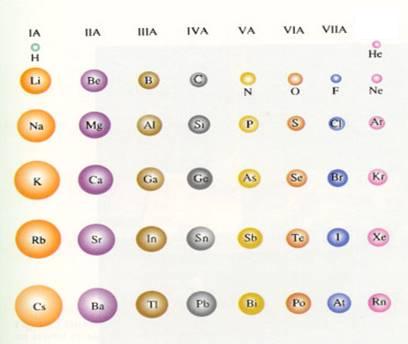
| The number of main levels can vary from 1 to 7, what coincides with the seven periods of the Periodic Table. | The number of valency electrons can vary from 1 to 8, what coincides with the number of main groups of the PT. |
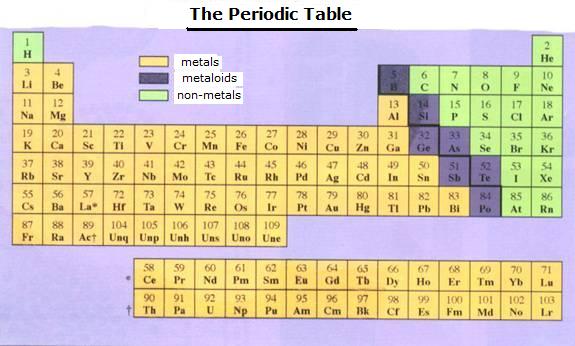
| I | II | III | IV | V | VI | VII | VIII | |
| 1 | H | He | ||||||
| 2 | Li | Be | B | C | N | O | F | Ne |
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar |
| 4 | K | Ca | Ga | Ge | As | Se | Br | Kr |
| 5 | Rb | Rb | In | Sn | Sb | Te | I | Xe |
| 6 | Cs | Bi | Tl | Pb | Bi | Po | At | Rn |
| 7 | Fr | Ra |
| metals | Cs |
| metalloids | Po |
| non-metals | Se |
| halogene |
|
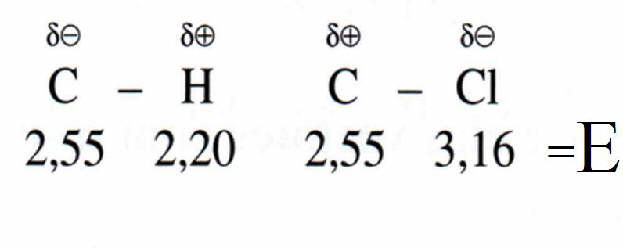
Electronegativity is the tendency of a (neutral) atom to attract negative charge (electrons).
|