C(s) + O2(g)Obviously all other substances present in the coal, will also be burned. Think of Sulphur and Nitrogen, who can, burning, can produce: NO, NO2 and SO2. These oxydes are delevered to the atmosphere and cause acid rain.CO2(g)

2C(s) +O2(g)2CO(g)
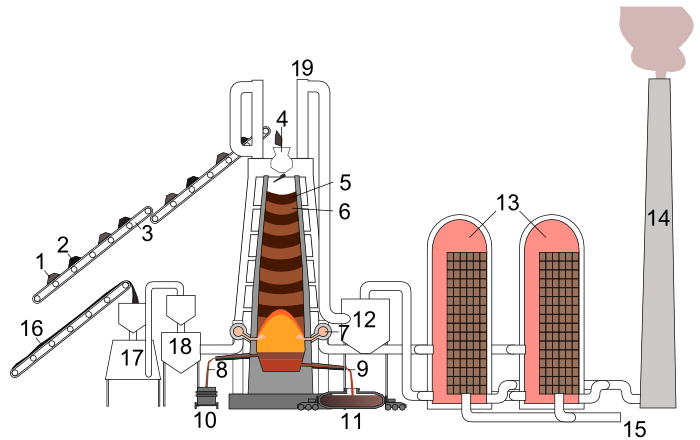
2C(s) + O2(g)Oxygen from the air will burn the carbon of cokes and the powdered coal; carbonmonoxyde (CO)is produced. This gas with a temperature of about 2200-2400°C will rise through the different layers of cokes and ore.2CO(g)
Fe2O3(s) + 3CO(g)3 CO2(g) + 2Fe(l) (Reduction of iron ore into iron)

Fe3+ (present in ore) + 3 e-Those electrons are delivered by the reductor CO, that in this proces will change into CO2. About that more:Fe (first in the liquid, later in the solid iron)
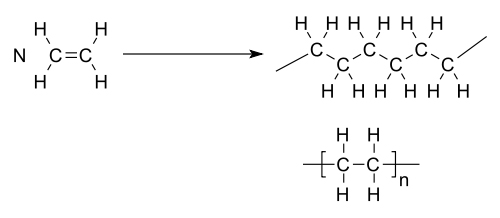
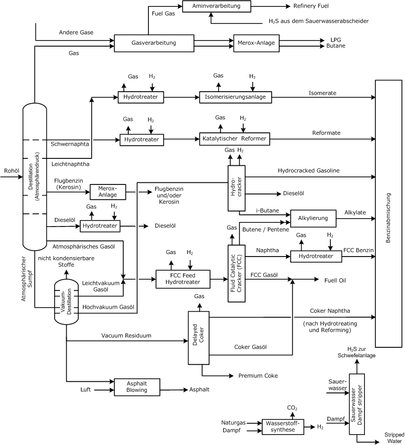
|
per molecule |
||