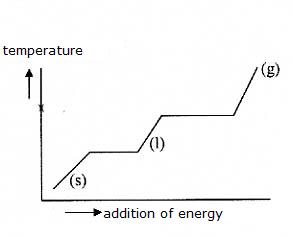
(s) |
(l) |
(g) |
|
(s) |
(s) + (s) | (s) + (l) | (s) + (g) |
(l) |
(l) + (s) | (l) + (l) | (l) + (g) |
(g) |
(g) + (s) | (g) + (l) | (g) + (g) |
| bonding distance | dipole moment | |
| HF | 90 x10-12m | 6,4 |
| HCl | 130 x10-12m | 3,5 |
| HBr | 140 x10-12m | 2,7 |
| HI | 160 x10-12m | 1,4 |
Apart from amorph Carbon, there are two well known lattice structures:
You can get artificial diamant from graphite. |
| (s) |
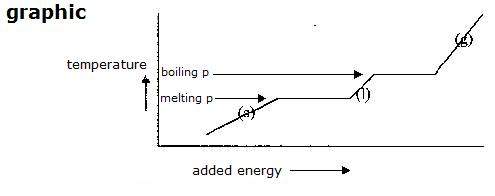
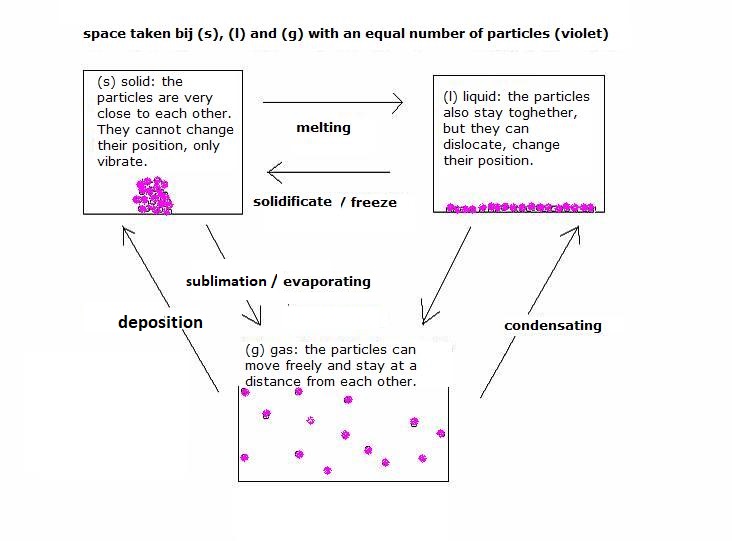
melting, solidification, freezing |
| (l) |
boiling or condensation |
|
(l) |
evaporation |
| (g) |
sublimation |
|
1. The LATTICE is the most important factor in determining a melting point.
How strong is a lattice? How easily can it be molten? Melting means that the lattice is destroyed, and it costs a lot of energy to destroy a strong lattice. The strength of an ionic lattice depends on the charges of the ions and of their mutual distances. In the lattice of CaO (with charges 2+ and 2-) wil melt more difficultly than the lattice of NaCl (with charges 1+ and 1-). Apart from that, also the ions of CaO are smaller (so: closer toghether) Small ions cause stronger lattices. They are closer toghether Salts, in general, will have high melting points. Also metalic lattices depend on charges and distances. Between the metals exist rather some differences: in general a metalic lattice will be strong (high melting point), but ther are exeptions: Mercury(l) is at normal temperatures a liquid. Lead, Tin, Lithium, Sodium, Potassium have no strong lattice. They melt easily. Extremely strong are the lattices of: Chromium, Wolfframium and Vanadium. Also see table V) |
|
2. VANDERWAALS-FORCES are the second factor in determining the melting points.
They exist in particular in molecular lattices, of which the strength depends on:
|
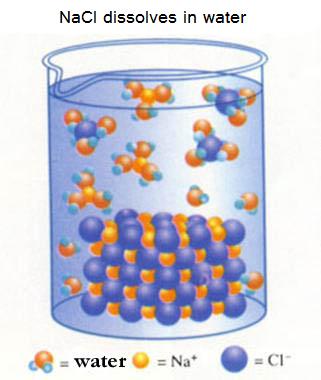
| Na+ | Cl- | Na+ | Cl- | Na+ |
| Cl- | Na+ | Cl- | Na+ | Cl- |
| Na+ | Cl- | Na+ | Cl- | Na+ |
| Cl- | Na+ | Cl- | Na+ | Cl- |
| Na+ | Cl- | Na+ | Cl- | Na+ |
| Cl- | Na+ | Cl- | Na+ | Cl- |
| kitchen salt(aq) | |
| distilled water | |
| chalk(s) | |
| Copper(s) | |
| tap water | |
| chalk water | |
| mercury |
| The purpose is that you investigate a number of substances according to the six items below. |
You receive a sample, not knowing what it is.
After every observation you must try to draw a conclusion. |
| The substances to investigate can be: | The six items of attention for every substance are: |
|
|

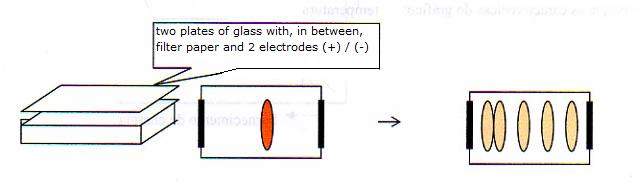
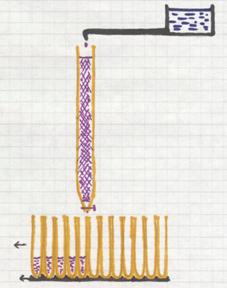
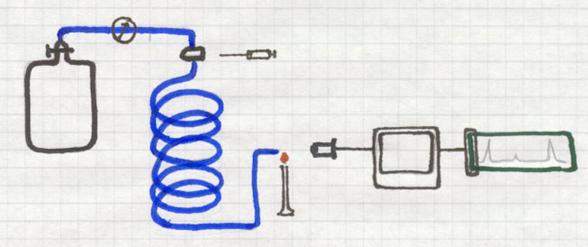
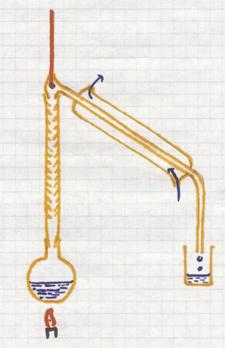


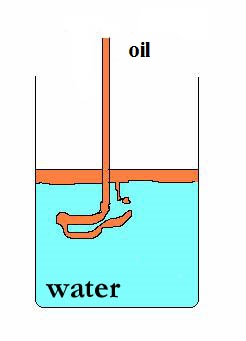
It deals with a homogeneous mixture of (l) + (s)
The image shows a method to evaporate carefully (on boiling water in a water bath).
Such a water bath can only serve as such if a temperature of 100ºC is enough to evaporate the liquid.
The dissolved substance will remain behind.
Question 56
Explain why the production of seasalt needs evaporation.