CH4 |
H2S |
NH3 |
H2O |
HCOOH |
HCl |
HCN |
|
H
| H - C - H | H |
H
/ S \ H |
H H
\ / N | H |
H
\ O | H |
OH
/ H - C = 0 |
H - Cl | H - C ≡ N |
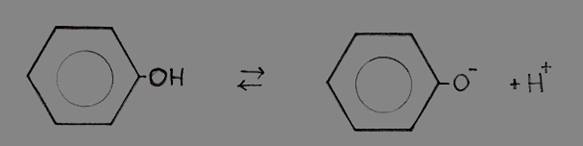
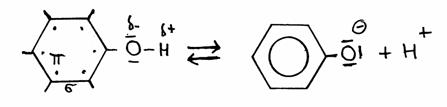
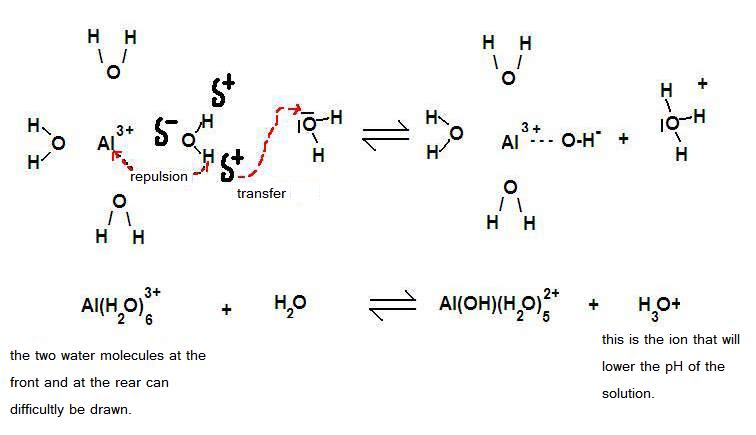
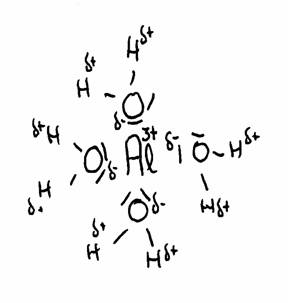
| Al(H2O)6 | + | H2O | Al(OH)(H20)5- | + | H3O+ | |
| acid | base | base | acid |
H2O |
+ |
H2O |
H3O+ |
+ |
OH- |
ΔH > 0 |
|||
| weak base | weak acid | strong acid | strong base |
| acid solutions | ||||||||||||||
| HA | + | H2O | H3O+ | + | A- | |||||||||
| acid | base | acid | base | |||||||||||
| A- is conjugated base of the acid HA | ||||||||||||||
| KA = acid constant | ||||||||||||||
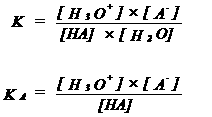
|
||||||||||||||
| If KA is very large (or pKA is very small), HA is a very strong acid | ||||||||||||||
|
An equilibriium always will shift to the side of the weaker ones. |
||||||||||||||
| basic solutions | ||||||||||||||
| A- | + | H2O | HA | + | OH- | |||||||||
| base | acid | acid | base | |||||||||||
| HA is conjugated acid of the base A- | ||||||||||||||
| KB = base constant | ||||||||||||||
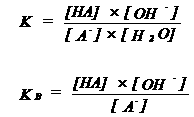
|
||||||||||||||
| If KB is very large (or pKB is very small), A- is a very strong base | ||||||||||||||
|
An equilibriium always will shift to the side of the weaker ones. |
||||||||||||||
| concentration | value of KA or KB | value of the pH of the solution | |
| A | 0.1M HCl | ||
| B | 0.1M HAc | ||
| C | 0.3M HN3 | ||
| D | 1M Na2CO3 |
| pH | -1 |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
| pOH | 15 |
14 |
13 |
12 |
11 |
10 |
9 |
8 |
7 |
6 |
5 |
4 |
3 |
2 |
1 |
0 |
-1 |
very strong and/or concentrated acid solutions |
acid solutions |
neutral solutions |
basic solutions |
very strong and/or concentrated basic solutions |
|||||||||||||


| phenolphtaleene | methyl red | |
| ammonia(aq) | carmin red |
yellow |
| HCl(aq) | colorless |
red |


two possible definitions of the buffer:
|
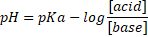
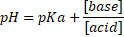
| where? | which enzyms? | the values |
| In the stomach | peptase, rennase en lipase | 1.5 - 4 |
| In the bowels | maltase, saccharase, lactase, ereptase | 6.6 - 8.5 |
|
I.
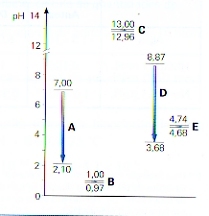
Period 2, Li - F |
Compounds with Hydrogen (hydrides) |
| LiH BeH2 BH3 {CH4} NH3 H2O HF | |
| Strong Base ---------------------weak base - neutral - weak acid | |
|
From left to right we see a gradual transition from base to acid,
where water is a real neutral substance, also CH4 for other reasons than water. The Hydride ion (H-) is is very basic and can be donated by LiH and BeH2. BH3 and NH3 have the tendency to catch H+, so they are weak basic, where NH3 is weaker than BH3. |
|
| Compounds with Hydrogen and Oxygen | |
| LiOH Be(OH)2 B(OH)3 = H3BO3 H2CO3 (=H4CO4) HNO3 [H2O] HFO3 | |
| Base ---------------------------amphoteric ----------- weak acid -------- strong acid | |
|
From left to right again you have a change from basic to acid,
where water also is a substance with Hydrogen and Oxygen, but that remains out of this analysis. The element B, connected with Oxygen and Hydrogen, can become Borium hydroxyde or Boric acid (can donate one H). Those two are similar. This substance we call an amphoteric substance or an ampholyte. |
|
|
II.
Period 3, Na - Cl |
Compounds with Hydrogen (hydrides) |
| NaH MgH2 AlH3 {SiH4} PH3 H2S HCl | |
| strong base --------------- weak base - weak acid - strong acid | |
|
From left to right we see a change from basic to acid,
with SH4 neutral for several reasons. The ion H- is very basic and can be donated by LiH and BeH2. BH3 and NH3 do have the tendency to catch protons, where NH3 is weaker than BH3. |
|
| Compounds with Hydrogen and oxygen | |
| NaOH Mg(OH)2 Al(OH)3=H3AlO3=HAlO2 H4SiO4=H2SiO3 H3PO4 H2SO4 HClO3 | |
| Base ---------------- amphoteric ------------ weak acid --------- strong acid | |
|
From left to right we see change from basic to acid.
The element Al, connected to Oxygen and Hydrogen, can deliver an amphoteric substance. |
|
|
III.
Group 1, Li – Cs |
Compounds with Hydrogen (hydrides) |
| LiH - NaH - KH - RbH - CsH (all are hydrides) | |
| Base --------------------- very strong base | |
| The ion H- is an ion with a very strong basic character. | |
| Compounds with Hydrogen and Oxygen | |
| LiOH NaOH KOH RbOH CsOH | |
| Base --------------------------- very strong base | |
|
All are strong bases because of the presence of the ion OH-
All are ionic bonds. The ion rays of the positive ions increase from top to bottom. |
|
|
IV.
Group 5, N - Bi |
Compounds with Hydrogen (hydrides) |
| NH3 PH3 AsH3 SbH3 BiH3 (All are hydrides) | |
| weak base --------------------- strong base | |
| The ion H- is an ion with a very strong basic character | |
| Compounds with Hydrogen and Oxygen | |
| HNO3 H3PO4 H3AsO4 H3SbO4 H3BiO4 | |
| strong acid --- weaker acid --- amphoteric --- weak base | |
| From top to bottom these substances lose their acid character | |
|
V.
Group 6 O - Po |
Compounds with Hydrogen (hydrides) |
| H2O H2S H2Se H2Te H2Po | |
| amphoteric ----- weak acid -------- strong acid | |
| The ion H- is an ion with a very strong basic character | |
| Compounds with Hydrogen and Oxygen | |
| H2O H2SO4 H2SeO4 H2TeO4 H2PoO4 | |
| amphoteric---- strong acid ------ weaker acids | |
|
VI.
Group 7, F - At |
Compounds with Hydrogen (hydrides) |
| HF HCl HBr HI HAt | |
| weak acid ------- strong acids | |
| Compounds with Hydrogen and Oxygen | |
| (HFO3) HClO3 HBrO3 HIO3 HAtO3 | |
| Does not exist strong acid ------- weaker acids | |
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The objective is that you investigate a number of acid base reactions, responding the 8 action points below. |
If possible you receive in a lab the needed substances. If not, you make the experiment only in your brain.
After every observation you must try to draw a (prelimanary) conclusion. |
| The 8 action poinst for every reaction are: | The reaction to investigate are: |
|
|
Which particles are present as reactants? (ionic formulas, molecular formulas, structural formulas)
|
|||||||||
| structural formulas | 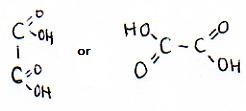 |
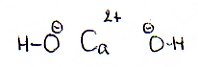 |
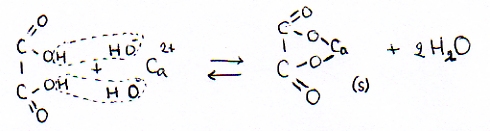
| Calcium oxalate(s) en water | |
| ionic formulas | Ca2+ en C2O42- (in een ionrooster) |
| molecular formulas | Ca2C2O4(s) en H2O |
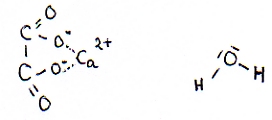
|
The objective is to make three buffer mixtures, and to control every buffer with the 6 action points (right under).
In order to do so, you can chose from the six substances below and weigh or measure them in the right amounts: |
If possible, you will receive in the lab the needed substances.
Hopefully you can use a lab. If not, then this is only a theoretical experiment. Write down you observations and conclusions. |
| The substances to be used: | The 6 action points for every reaction: |
|
|
|