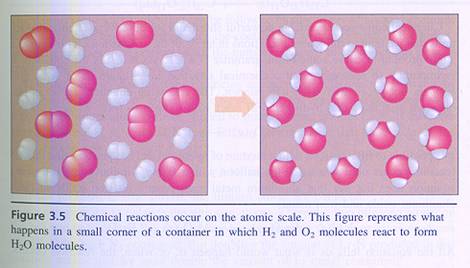
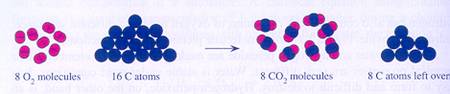
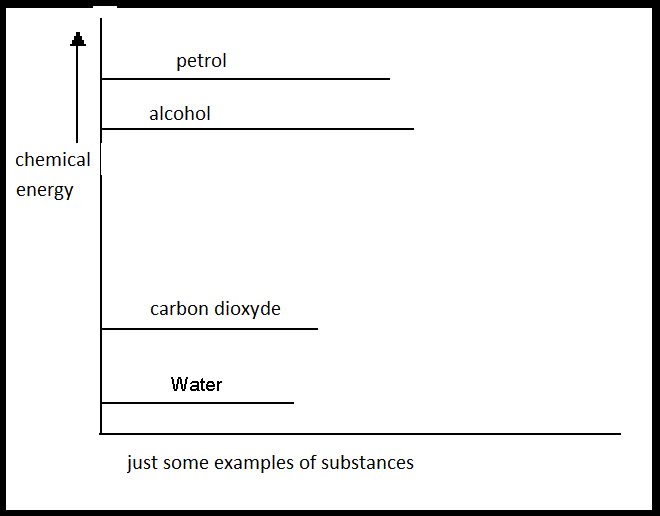
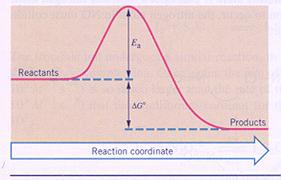
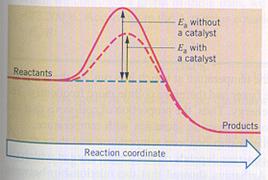
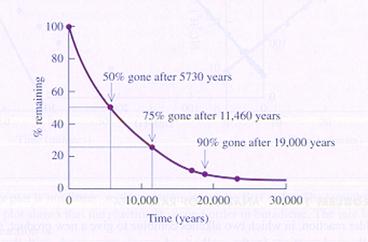
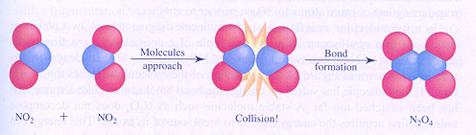
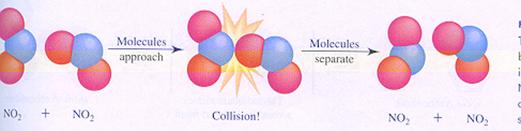
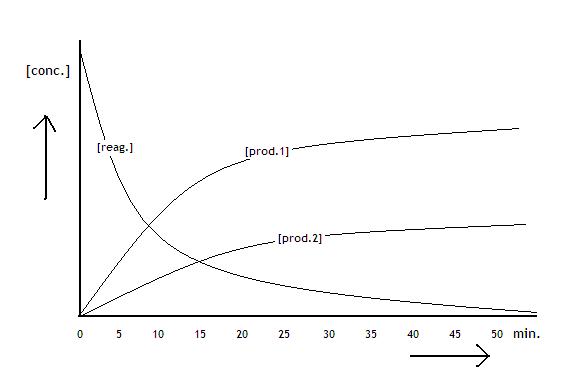
|
Important rules for resolving a reaction calculation: |
| 1 |
Use balanced reaction equation and add the phases. |
| 2 |
Underline the data, the given data and those asked for. The other substances (without any data) are not needed in the calculation.
Sometimes the data are directly given, sometimes indirectly.
The calculation is continues with only the underlined substances.
|
| 3 |
write down the mol proportion |
| 4 |
Where needed, convert the mol into the right units (to be found in the data and in the required answer) |
| 5 |
Introduce a conversion factor to arrive at the real amounts, given in the data.
That's how you finish the calculation.
|
Att.: It is absolutely necessary that you continues in every step with all (underlined) substances, to assure that you know what you are doing.
Only then the calculation remains a real thing, and not an abstractum.
| 5 |
in reality we do not have 16 grammes, but only 4 grammes for combustion.
To be introduced a factor of 4/16. (in this case we can simply divide by 4)
4/16 x 16 gramme CH4(g) produce 4/16 x 44 gramme CO2(g)
to finish: standard condition mean: at temp = 25oC and pressure of 1 atm. Then 1 mol gas = 22,4 liter
1/16 x 44 = 11 gram CO2(g) is produced,
that equals 4/16 mol = 4/16 x 22,4 liter CO2(g) = 5,6 liter
|