questions and answers
Q 01-03
Atoms with a very high atom number (>92) are no longer (to keep) stable. What could be the reason for that?
Answer
Atoms with a very high atom number also have lots of protons. In a stable nuceus, the neutrons succeed in holding de protons toghether in spite of their positive charge (+1).
However, if the number of positive charges (protons) become too many, this is no longer possible. Then, the close and large amount of neutrons can no longer prevent, overcome the repulsion between the protons.
Q 01-09
Noble gases have a similar electron division; Helium does not have 8 valency electrons, but is nevertheless a nobel gas. Explain that.
Answer:
The noble gases do not react because they have a 'full' outer orbital, saturated with electrons. Normally, they are 8.
Helium has only 2 electrons, but also only one main orbital. And that one is de first main orbital that is fully satisfied with 2 electrons; more this orbital does not want/cannot have.
So the first main orbital has only one level s, that contains a maximum of 2 electrons.
Q 01-17
Natural carbon is composed of 998,89% the carbon isotope 13C
The relative atom mass of the isotopes is 12.000 for 12C en 13.003 for 13C
Calculate the atom mass of this element.
Answer
The atom mass is an average of the masses of isotopes.
You must take into account the percentages of those isotopes.
So if there is a lot of 12C and very few 13C, the atom mass will of course stay closer to 12 than to 13.
98,89 % of Carbon is 12C, so: 1,11 % is 13C
The contribution of 12C at the total atom mass is: 0,9889 x 12,000 = 11,87
The contribution of 13C at the total atom mass is: 0,011 x 13,003 = 0,14
So the average atom mass of Carbon will be: 11,87 + 0,14 = 12,01
Q 02-06
- Define the position of atom number 18 in the PT and give also the electronic configuration.
- idem for number 23.
- An element has position in main group V and in Period 3. What is its atom number?
Answer:
a.
atom number 18
electronic configuration = 1s2 2s2 2p6 3s2 3p6 = 2 - 8 - 8 (main division)
with 8 valency electrons, it must be a noble gas, so: main group 8.
ending with p-level, the element must belong to a p-block.
with three main orbitals, this element must be in the third period.
b.
atom number 23
electron configuration = 1s2 2s2 2p6 3s2 3p64s23d3 = 2 - 8 - 11 - 2
with 2 electrons in de outer main orbital, this must be a metal, but not from second main group; de configuration ends in a d-level.
so it is an element from the d-block
there are four main orbitals, zo it is an element from period 4
c.
main group 5 ends in s2 p3 having 5 electrons in the outer main orbital.
the third period indicates three main orbitals, so there is only one possible electron configuration: 1s2 2s2 2p6 3s2 3p3 = 2 - 8 - 5
because there are 2+8+5 = 15 electrons in the orbitals, the nucleus must contain 15 protons.
the atom number is 15.
Q 02-11
Explain why Fluorin the most reactive halogene is.
Answer:
Fluorin stands in the seventh main group at the top. This means automatically that F has the highest electronegativity of all halogenes. Reason for that is that, compared with clorine and other halogens, the atom of F has the smalles atom ray (only 2 main orbitals) and so
the positive nucleus will attract the negative charge (electrons) very strongly.
The neutral atom as such maintains this attraction power to any negative charge nearby.
This is the property that makes fluorine so reactive.
Q 02-13
Is the following affirmation true or false?
"The noble gases have a very low ionization energy"
Explain your answer.
Answer
The noble gases have a great stability, so it won't be easy to change their electronic structure.
Remove electrons and form positive ions will cost extremely lots of energy.
So the affirmation above is not true. On the contrary.
Q 02-16
Normally an atom prefers 8 valency-electrons. Based on that, give the electronic configuration of the following molecules/ions:
F2 CO N2 HS- OH-
Also explain if you expect polarity in this particle.
Answer:
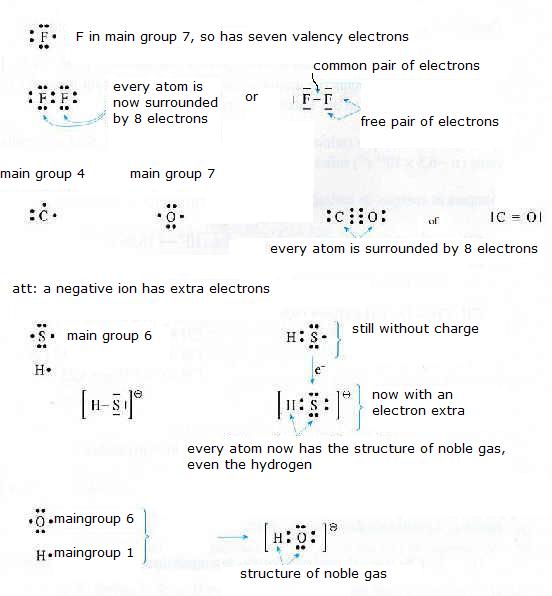
In F2, in CO, in N2 no or litle polarity can be expected, because there is no or little difference in electronegativity of the connected atoms.
But in HS- and in OH- you may expect polarity.
Q 02-17
The molecule of dicloromethane is polar? Explain your answer.
Answer:
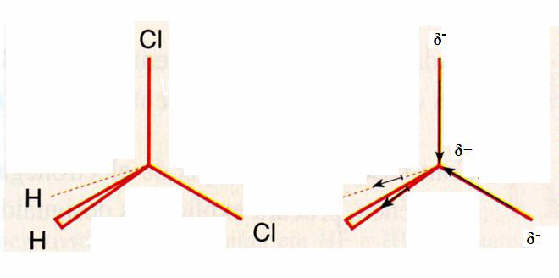
In the above figure you can see dat the negative charges are situated at one side of the molecule. this molecule will have a two-polar character. The difference between electronegativity of Carbon and Clorine is big enough to be effective.
Q 03-10
Check in every structure:
- is the total number of valency electrons right, and
- have the atoms realised a stable electronic configuration.
H /O\ H /O\
/ || | // _
H - O| S H - C - C - O - H
/ \ |
|O| |O| H
Answer:
All atoms, except H, have four dashes around, i.e. 8 valency electrons. The H-atoms have one dash = two valency electrons.
So: yes, all atoms have reached a stable situation.
Q 03-19
a molcule of water has no linear structure, but is kind of triangle. This small difference has great consequences in our world.
Describe shortly those consequences.
Answer:
Suppose a watermolecule would be linear: the two H atoms and the O atom all on one line. Than this symmetrical molecule as a whole would not be polar, would not be en dipole.
The attraction between de water molecules would be less than between the triangle molecules.
With much less attraction, these watermolecules (very small and light) would be no longer a liquid, but a gas, just like carbondioxyde.
conclusion: there would not be any see, river, rain, no life at all.
Q 03-25
Mention a substance (+ formula) with two metals and one non-metal, and with two bonding types (ionic and covalent).
Answer:
This could be, for example: K2Cr2O7.
There is an ionic bond between K+ and dicromate Cr2O72-
Thes substance has also covalent bonds between Cromium and Oxygen (in a complex ion).
Q 03-27
Molecules of the Halogenes have relatively low boiling points, that can differ a lot.
Explain those differences, using the tables (tabel V).
Answer:
Some data from tabel V about boiling points: (column 7, in Kelvin!!)
1 = element |
2 = symbol |
3 = atom- number |
4 = mass- number |
5 = electro- negativity |
6 = melting point |
7 = boiling point |
8 = atom ray x 10-12m |
9 = ion ray x 10-12m |
10 = vanderwaals ray x 10-12m |
Fluorine |
F |
9 |
19,0 |
4,0 |
54 |
85K= -152oC |
64 |
133 |
135 |
Chlorine |
Cl |
17 |
35,5 |
3,2 |
172 |
239K= -152oC |
99 |
181 |
180 |
Bromium |
Br |
35 |
79,9 |
3,0 |
266 |
332K= -34oC |
114 |
196 |
195 |
Iodine |
I |
53 |
126,9 |
2,7 |
387 |
458K= 59oC |
133 |
219 |
215 |
Astatium |
At |
85 |
2,2 |
575 |
610K= 185oC |
140 |
Relatively, those are low temperatures (compared with salts for example) because the intermolecular bonds are weak (there is no polarity)
The differences come from the fact that the molecules have increasing mass (see column 4)
Heavier molecules have more difficulties in becoming gas; the vanderwaals forces increase.
Q 04-08
the molecule of sulfuric acid has a structure formula. Give a possible structure formula.
Answer:
The molecule formula is: H2SO4. So: 1 molecule contains 2 atoms H, 1 atom S en 4 atoms O.
-
one hydrogen atom contains one (valency) electron.
total: 2 -
one sulfur atom contains 6 (valency)electrons
total: 6 -
one oxygen atom contains 6 (valency)electrons
total: 24 -
one molecule of sulfuric acid contains
a total of 32 valency-electrons = 16 pares / dashes.
- Always avoid connecting equal atoms (exect C); so don't connect two O-atoms.
- The hydrogen atoms have only one bond, zo always have a position at the outer side of the molecule.
- The sulfur atom must be in the centre.
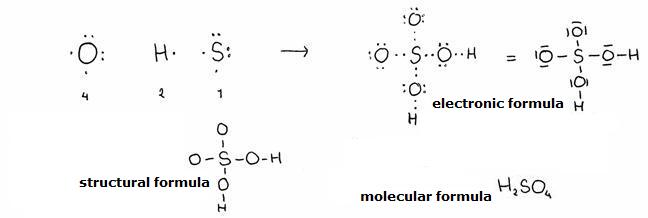
Control the total amount of valency electrons in the electronic structure. Didn't we lose any electrons?
Q 04-09
Explain the acid character of the ammonium-ion, writing only a reaction equation.
Answer
In water:
NH4+ + H2O
The ammonium-ion releases a H+ ion (to water) and every substance that releases H+ is an acid.
This solution contains more H3O+ than normal, neutral water.
Q 04-17
which bonding types are in a propene molecule?
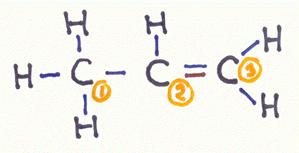
Answer:
C1
has four bondings of the type σ
(linear overlap of the sp3-hybride: 3 with s1-orbitals of the H-atom and 1 met sp3-hybride of C2
C2
has 3 bondings of the type σ (linear overlap of the sp2-orbital: 1 with the 1s1-orbital of the H-atom, 1 with the sp3-hybride of the C1 and one with the sp2-hybride of the C3)
has 1 bonding of the type π (parallel overlap of the 2pz-orbital with the 2pz-orbital of the C3)
C3
has 3 bondings of the type σ (lineare overlap of the sp2-hybride: 2 with 1s1-orbitals of the H-atoms and 1 with the sp2-hybride of the C2)
has 1 bonding of the type π (parallel overlap of the 2pz-orbital with the 2pz-orbital of the C2)
Q 04-19
Answer the following questions about the structures A, B, C e D:
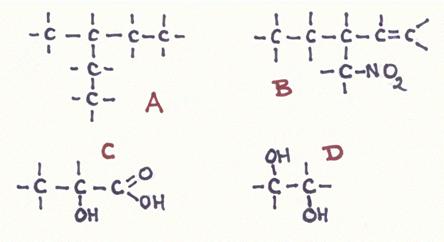
- Give the official name of every structure;
- How many secondary C atoms has every structure?
- Does any of the structures has a secondary OH-group?
- Is in all cases the main chain also the longuist chain?
You must know well that the main chain - in general - will be the longest; but not always you can chose the longest as the main chain.
for example: if there is a double bond or a functional group in the structure, the main chain must contain that double bond or that functional group.
In B we see a chain with 3 C-atoms (not the longest), but must be the main chain because there is a double bond and a nitro group.
The name of B becomes: 1 butene (main name), with a branch (ethyl) and a functional group (nitro)
The name: 4-nitro, 3-ethyl, 1-butene
The numbering could also be: 1-nitro,2-ethyl, 3-butene Explain that.
Structures on paper can be confusing because they are not tridimensional.
The model of A shows clearly that no confusion is possible about the branches and that the branche methyl is. The name is: 3-methyl pentane.
A C-atom, connected with only 1 other C-atom is called a primary C-atom
Structure A contains 2 secondary C-atoms and structure B the same. Explain that.
Structure C has 1 secondary OH group connected to the secondary C-atom.
The trivial name of structure C is 'lactic acid', but this is not the official name. The main chain has 3 C-atoms with only single bonds: propane.
The one with the carboxyle group automatically is C-atom number 1. So the OH-group is at number 2.
The official name must be: 2 hydroxy propanon acid.
Structure D is a diol, with two alcohol groups. The main chain contains only two C-atoms without double bonds, so:
the main name must be ethane, with two groups OH.
Names: Ethane 1,2-diol
or:
1,2-dihydroxy, ethane.
Every functional group or every branch requires its own number..
Q 04-24
Give the name of the following chemical structure, and check the models. Are they right?
(no need to attend the colours of the models. The pictures do not show the usually used model colours).
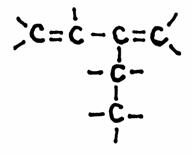
Answer:
The structure has a chain of 5 C-atoms and should start with: penta..... in the main name.
However, there are double bonds to be put in the main chain.
So, the main chain must include those double bonds and has only vour C-atoms; the main name starts with but......
A branche (side chain) of two C-atoms will get the name ethyl
If the main chain had only One double bond, the name would be butene, but there ar two double bonds: butadiene
Also must be explained where those double bonds are positioned in the main chain.
The two double bonds are connected to the C-atoms 1 and 3: 1,3 butadiene
The branche is connected to C-atom 2 (or 3, but you should always try to number as low as possible): 2 ethyl 1.3 butadiene
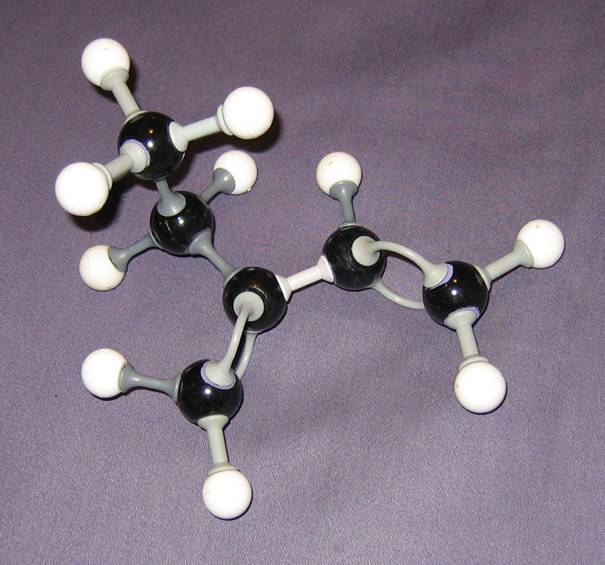
About the models:
In principle all models are okay. The first shows the hydrogen atoms, the second only the bonds to those hydrogen atoms and the third shows just the 'skeleton' of the molecule.
On paper it is difficult to draw threedimensional structures, but good modles show you the directions of the atoms.


Q 04-28
Give the official name of Valine (one of the amino acids)
Answer:
The structure of Valine:
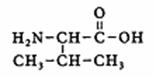
We recognize a main chain with 4 C-atoms and one carboxylgroup:
main name: butanoic acid
+ an amino group at position 2
+ a methyl group at position 3
So: the official name must be: 2-amino, 3-methyl, butanoic acid
Q 04-32
Have a close look at the following structures:
1)
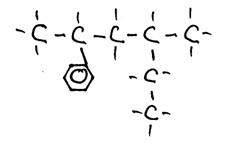
2)
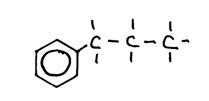
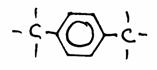
3)
- Give the name of every structure. If possible, give another name.
- The structures 2) and 3) are isomeres? Yes of No? Explain your answer.
a)
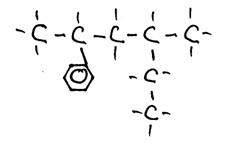
2-fenyl, 4-methyl-hexane
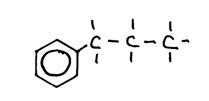
1-fenyl-propane; or: propyl-benzene

1,4-dimethyl-benzene or: para-dimethyl-benzene (= p-dimethylbenzene)
att:
In structure 1) you chose the main chain with the highest number of C-atoms (=6); you can consider Benzene as a functional group.
In structure 2) number 1 may not fail; there exists also 2-fenyl propane. so please: 3-fenyl propane is wrong! (why?)
Benzene can be a functional group, but als considered as main chain.
b)
The empiric formulas of the three substances are different. There is no isomery.
Q 05-03
Imagine that you have a substance composed of very movable particles: very tiny, light and with little attaction power.
This substance will be liquid, solid or gas? Explain your answer.
Answer:
Those particles will attract each other badly, zo they stay easy away one from another. Only with lots of difficulty it will be possible to join them and to keep them toghether; i.e. only at very low temperatures and very high pressure.
Be sure that this substance is a gas under normal conditions.
Q 05-05
Do you think that the total number of people on earth is more or less than 1 MOL? Make an estimation.
Answer:
Suppose the total number of people on earth is about 8 billion.
That is: 8.000.000.000 = 8 x 109
1 MOL = 600.000.000.000.000.000.000.000 = 6 x 1023
1 MOL is much more than 8 billion. So on earth the number of people is not at all 1 MOL. Be happy with that!!
Q 05-10
True or false? Explain!
12 g of Carbon contains 1 mol of atoms
Answer:
The element Carbon has, according to the table, an atomic mass of 12.
On micro scale, this means that the nucleus contains 12 nuclear particles (6 protons + 6 neutrons)
On macro scale, this means that 1 MOL Carbon atoms weigh 12 x 1 grams
So the above affirmation is true.
Q 05-20
Please consider beer as a mixture of water and alcohol. What kind of mixture is this?
Answer:
Both alcohol and water are liquids at room temperature. Both are polar substances, so they will mix very well and homogeneous.
This is a homogeneous mixture of (l) + (l)
Q 05-23
Suppose that you broke bottle of 1 liter of beer. The beer had a volume percentage of 6 (so this is heavy beer). Density of alcohol is 0.8
How many molecules of alcohol did you drop?
Answer:
One liter = 1000 ml and 6 volume percentage of that is 6 x 10 = 60 ml
So: 60 ml of pure alcohol fell on the floor.
The density is 0.8 g/ml → 1 ml alcohol = 0.8 gram alcohol → 60 ml alcohol = 60 x 0.8 = 48 grams of alcohol
conclusion: 48 g of alcohol was dropped on the floor.
The molecular mass of alcohol = 2xC + 6xH + 1xO = 24 + 6 + 16 = 46.
This means that 46 g alcohol = 1 MOL alcohol = 6 x 1023 alcohol molecules
You see immediatly that 48 g is just a littlebit more than 1 MOL. To be exactly: 48/46 times more:
After all, 48/46 x 6 x 1023 molecules of alcohol were dropped on the street = 6,26 x 1023 = 626.000.000.000.000.000.000.000 molecules. Isn't that a lot of them?
At last, you better drop them all on the floor of in the street, that allow them to destroy your brain cells.
Q 05-39
The following substances must be put in order of increasing boiling point:
water, nitrogen, hexane, 2.3-dimethyl butane, glycerol.
Answer:
You must check those substances on the next properties: (in sequence of importance)
- Charge forces between the particles (are they ions or dipole molecules?)
- vanderwaalsforces; for that you must compare M (the molecular masses)
- the three dimensional shape of the particles.
Hardly boil means: higher boiling point.
The substances with the higher boiling points are water and glycerol.
So, the substances with lower boiling points must be Nitrogen, hexane and 2.3-dimethylbutane.
- Nitrogen is lighter than hexane and 2.3-dimethylbutane
- hexane and 2.3-dimethylbutane have equal masses
- water is much lighter than glycerol
hexane has a longer shape, is more linear than 2.3-dimethylbutane;
2.3-dimethylbutane has a more spherical shaped molecule (with branches) and so has easier opportunity to escape from the liquid → lower boiling point.
The final sequence of increasing boiling point must be:
Nitrogen - hexane - 2.3-dimethyl butane - water - glycerol
Q 05-42
affirmation: "During evaporation, solid I2 will produce loose Iodine atoms."
True or False? Explain!
Answer
Iondine has the molecular formula I2, i.e. every molecule is built up of two atoms (connected via a covalent bond)
Evaporation means the following process:
I2(s) → I2(g)
Evaporation is not a chemical reaction: the covalent bonds are not broken. Just the intermolecular bonding (in this case vanderwaals forces) will be broken.
The molecules of I2 themselves did not change.
And no loose atoms are formed.
Q 05-50
Consider the possibility to separate a (g) + (g) mixture in the components via paper cromatography. Explain the situation.
Answer
A mixture of different gases can only be investigated/separated in a closed space, so the gases cannot escape.
Also, you must force all gas particles move
Paper chromatography takes often place in a closed space, but does not count with the evaporation of the substances to investigate. This device is much too open for gases. Paper chromatography works on basis of a running liquid, where gases are moved on a running gas.
It will be very difficult of even impossible to separate a mixture of gases into the components by means of paper chromatography.
Q 05-60
In our body energy rich substances are converted into products. Are we here dealing with exothermic or endothermic reactions?
Answer
If our body transforms (in the metabolism) nutriciants via chemical reactions, than the main objective is to provide the body with energy. If you eat, you get energy. The important nutriciants, those with lots of energy, are sugars, oil, fats.
Proteins do not serve for energy provision, but to provide te body with building bricks of new important body substances.
So energy rich substances (like sugars) are converted into energy poor substances (like water en carbondioxyde). The energy that becomes available, can be used by the body for other happenings that cost energy. Such a reaction that liberates energy is of course an exothermic reaction.
Q 06-05
What is the most important property of the water molecule that is responsible for the liquid state of water? (knowing that the water molecule is very small and light)
Answer:
Take a molecule just as small and light, for example ammonia or natural gas. Why is it that those molecules stay gaseous and water not?
The reason must be found in the strong polarity of the water molecule.
One side of the water molecule (with the H-atoms) has rather positive charge where the other side (with the O-atom) has a rather positive charge.
If such molecules come toghether, the negative side of one molecule will attract the positive side of another one. There is strong attraction power between water molecules and it will be difficult to separate them.
Formation of gas / evaporation means that the water molecules must separate. That will cost lots of energy/heat to make liquid water gaseous, to evaporate it, to boil it.
Without that polar character of the water molecule, water would not be a liquid at all; there wouldn't rivers, rain, oceans. We wouldn't have a world like ours. We would not exist.
Q 06-11
In the nineteenth century arose a special theory about fire. They introduced a new concept: "flogiston". Flogiston should be a very volatil substance, escaping from burning matter.
Later this theory was rejected because of experiments. With those experiments the mass was determined of the substances before and after the combustion. Observations did not confirm such a thing as 'flogiston'. on the contrary.
Try to present this rationalisation.
Answer:
Suppose research was done with 5 gramms of wood. It was put in a porcelain and continuous weighted, also during the combustion. You understand that only ashed remained at the end.
The mass of those ashes were much less dan the 5 gramms, so it was not that crazy to believe that something disappeared during the process.
But nowadays, we know that gaseous substances are formed (even is you can't see them). In this cas lots of carbondioxyde and water vapor will be produced.
They repeated the experiment in such a way that all products (including carbondioxyde, water and ashes were collected and weighted. What appeared?
The mass of the products was not less, but even more thar the 5 gramms. The products are heavier than the original 5 gramm of wood. conclusion: no substance disappeares, but new substance is added!
For a moment the idea came up that the escaping substance could have a negative mass, but most scientists didn't like that thought.
Later was discovered that in the combustion, Oxygen is the other reactant that participates in the reaction.
If you measure the 5 gramms of wood + the needed oxygen, and you compare that tot the total mass of the products, than you find that the two masses are equal.
Q 07-02
Dissolving a salt in water, is that a physical or a chemical process?
Answer
This is not a real chemical reaction; the substances do not really change; there are no new products. So you could say: physical. Een echte scheikundige reactie is het niet, de stoffen veranderen niet echt, er zijn geen nieuwe producten. In die zin zou je zeggen: natuurkundig.
But completely without chemistry you cannot. The ions (of the ionic lattice) move away from each other and the free coming ions are immediatly surrounded by water molecules.
You see, it is not always 100% clear.
Q 07-06
A macro molecule can react with a small ion in the mol-proportion 1 : 1. Estimate the mass proportion.
Answer:
Suppose the macromolecule is built up of zo many atoms that teh molecular mass is, for example, 20,000. This is a realistic option.
The small ion can, for example, have mass of 40 (could be Ca2+).
With a molproportion 1 : 1 the mass proportion is 20.000 : 40 = 500 : 1
Half a kilogram of the macromolecular substance reacts with one gram of ion.
Q 07-15
How mucht (mass and volume) carbon dioxyde will be produced at the complete combustion of 4.01 g of methane?
Answer:
| How mucht (mass and volume) carbon dioxyde will be produced at the complete combustion of 4.01 g of methane? | |
| 1 | CH4(g) + 2 O2(g) |
Therefor me need to introduce a factor: 4/16. (accidently in this case we can divide by 4)
4/16 x 16 gram CH4(g) produce 4/16 x 44 g CO2(g)
finally: standard circomstances include: at temperature of 25oC and a pressure of 1 atm. Then 1 mol gas = 22,4 liter
1/16 x 44 = 11 gram CO2(g) is produced,
that equals 4/16 mol = 4/16 x 22,4 liter CO2(g) = 5,6 liter
Q 07-22
3 mol Chlorine and 150 grams of Iron are joined and produce Iron(III)chloride.
Which one is the limiting reactant? in other words: which reactant will be consumed completely?
Answer:
| 1 |
the right equation: 2Fe(s) + 3Cl2(g)
|
| 2 |
There are data about Fe and Cl2 and about iron(III)chloride is no data or question, so the calculation only deals with the two reactants.
2Fe(s) + 3Cl2(g) |
| 3 | MOL-proportion is: 2 : 3 or better: 3 mol chlorine react with 2 mol iron. |
| 4 |
The Chlorine has been given in mol, so no unit conversion is needed.
The iron is given in grams: 3 mol chlorine react with 2x55,8 = 111,6 gram iron (see also tabel V) |
| 5 | In reality there are 3 mol chlorine and 150 grams iron. So iron remains. The chlorine is the limiting factor. |
Q 07-23
Nitrogen and Hydrogen react in a direct reaction and produce ammonia in an industrial reactor with catalyst.
1000 grams op product (ammonia) are formed with a yield of 97.8 %
The molecular mass of ammonia is 17,0
How many mol of reactants were needed?
Answer:
| 1 |
The complete reaction equation:
N2(g) + 3H2(g)
|
| 2 |
We must calculate the reactants and we have data of the products, so all substances participate in the calculation:
N2(g) + 3H2(g) |
| 3 |
Mol proportion: 1 : 3 : 2 or rather:
3 mol of Hydrogen reacts with 1 mol of Nitrogen and produces 2 mol of ammonia. |
| 4 |
3 mol Hydrogen reacts with 1 mol Nitrogen and forms 2x17 = 34 g ammonia
out of the 1000 g product, 978 grams are pure ammonia; so 978 g of ammonia are formed in stead of 34 grams. The correction factor must be 978/34 = 28,8. |
| 5 | at last: 28,8x3 = 86,4 mol Hydrogen reacts with 28,8x1 = 28,8 mol Nitrogen and forms 28,8 x 34 = 1000 gram 97,8% ammonia. |
Q 07-25
The condensation of water vapor is exothermic or endothermic? Explain your answer.
Answer:
Condensation means that the gaseous watermolecules (moving loose, not toghether) now come toghether, join, and become liquid water.
Joining, according to the basic rule, is always exothermic. Condensation must deliver energy; the environment becomes warmer.
Q 07-27
The bonding energy of the Nitrogen molecule is very hight. Why is that?
Answer:
The bonding between the two N-atoms is triple. It will cost lots of energy to break such a triple bond (and making such a bond will deliver lots of energy).
Check this in the table VI tabel VI.
Q 07-34
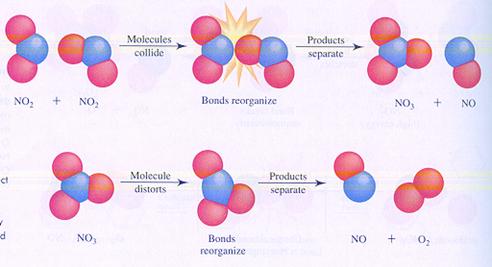
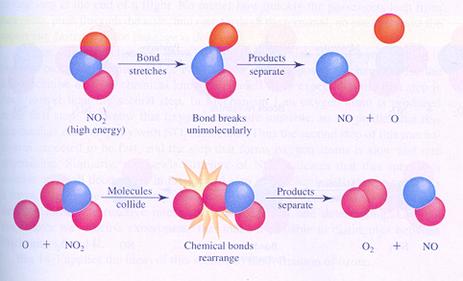
Consider two possible reaction mechanisms, or different steps of the reaction between molecules of nitrogendioxyde
In case I, two molecules collide and that is the start of the reaction.
In case II, one molecule will split up and one product collides with another molecule.
compare the two mechanisms and search the differences.
case I
case II
Answer:
Both reactions suffer a collision between two particles, but at different moments in the mechanism.
There is no information about the rate of the reaction
So is will be difficult to compare the two rates of the two mechanisms.
In both cases, No is formed earlier than O2.
Q 07-36
Why is it impossible to keep the concentration of the reactants constant during a reaction?
Answer:
The reactants react during a reaction!!! so, automatically the concentrations will decrease.
Unless, of course, you add new reactant during the reaction continuously.
Q 07-45
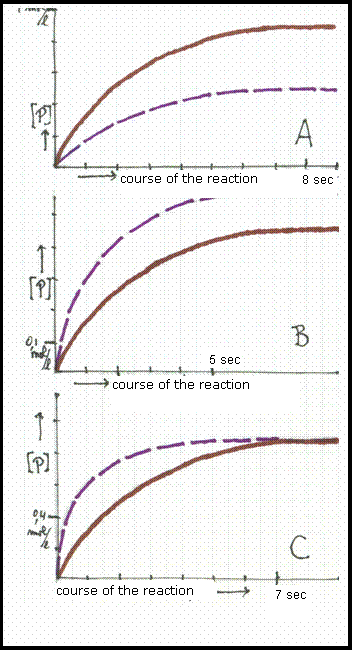
The three diagrams show graphics:
'concentration of the product in mol/l' versus ‘course of the reaction in seconds’.
The two graphics differ only in temperature in such a way that the non interrupted line shows the reaction at the lowest temperature.
Chose the right diagram and explain your choice.
Answer:
Diagram C shows the right graphic. Eventually the amount of product is equal. Working at higher or lower temperature only will influence the rate at which the products are formed. The non interrupted line shows that at higher temperature the products are produced in less time.
Q 07-47
- We can divide all chemical reactions in composition reactions and decomposition reactions. Explain that.
- You can also divide the reaction in another way, also in two kinds. How?
Answer:
In every reaction, bondings are broken and new ones are made. If particles participate in a reaction, they first must be free to enter a new bond.
The other way is of course the division in exothermic and endothermic reactions.
Q 08-07
Some equilibria in the human body - in this case in the blood - are:
- HHb + O2
 HbO2 +H+
HbO2 +H+
- CO2 + H2O
 H2CO3
H2CO3
- H2CO3
 H++ HCO3-
H++ HCO3-
What kind of problems can suffer a person with a low pH-value of blood?
Answer:
Suppose, someone has such a low pH-value in the blood. Or: his blood is a bit more acid than normal. There are more H+-ions than normal.
The equilibria i and iii will notice that immediatly and deslocat to the left side (according to the rules of Chatellier and Van’tHoff).
In case iii extra H2CO3 will be produces, which, in its turn, will influence equilibrium ii, so that one goes even more to the left. The consequences are:
- The hemoglobine will capture less Oxygen
- The blood will transport less carbondioxyde
And in case of very serious lack of oxygen / overdosis of CO2, he can die.
Q 08-12
Is it true or false? explain your answer.
"If an enzym is influencing an equilibrium, the value of the equilibrium constant K will change"
Answer:
An enzym may be considered as a 'bio-catalyst'.
And you have learnt that a catalyst never has any influenct on the value of K, so the same for an enzym.
The above affirmation is false.
Q 08-17
What is the value of K:
- In the case of very strong products and very weak reactants?
- In the case of very weak products and very strong reactants?
Answer:
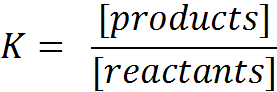
"Strong products" means that those products have a strong tendency to react and thus to reproduce the (weak) reactants.
In this situation, if the equilibrium is reached, the product concentration will be low and the reactant concentration high.
In the formula for K the quotient will have a very low value.
In the opposite situation, of course, the conclusion will be the opposit: K has a very large value.
You can say: An equilibrium with lots of products will have a large K-value, and in case of few products, the K-value will be small.
Q 08-24
Affirmation: "Pure boiling water (so at a temp of 100 oC) has no pH=7"
Explain why this theses is true or false.
Answer:
The water equation has - as all equilibria - a thermodynamic effect.
Meaning that the dissociation of water will cost energy; the opposite will deliver energy.
Heating will stimulate the endothermic reaction, i.e. the dissociation. (Dissociation tries to obstruct the raise of temperature).
So at higher temperatures, water will contain more hydronium ions (H3O+) and more hydroxy ions (OH-)
More ions H3O+ means automatically, according to mathematics, that the pH value will decrease.
The above theses is true.
Q 09-01
Neutralisation of diluted cloric acid with a solution of sodiumhydroxyde can be represented as follows:
H+(aq) + OH-(aq)  H2O(l)
H2O(l)
Is that true or false?
Explain your answer.
Answer:
No matter what is the acid, it can supply H+-ions, certainly cloric acid can.
Sodium hydroxyde dissolved in water is dissociated in ions, Na+ and OH-. These ions do not undergo any change at all.
The only change is the reaction between H+(aq) with OH-(aq), producing water.
The affirmation is true.
Q 09-06
Choose the right answer and explain your choice.
- does not contain salts
- Has equal amounts of H+ and OH-
- has no H+ and no OH-
- does not contain acid or base
1. Salts are composed of ions, and those ions can have an acid of basic character, but not necessarily.
2. A solution in water has always some ions H+ and OH-
So: A neutrol solution at a temperature of 25º Celcius must have equal amounts of H+ and OH-
Q 09-07
- Next substance will be an acid of not an acid? in other words: will the particle be able to donate protons?
- In your answer, try to include the polar character of the molecule bonds.
CH4 |
H2S |
NH3 |
H2O |
HCOOH |
HCl |
HCN |
|
H
| H - C - H | H |
H
/ S \ H |
H H
\ / N | H |
H
\ O | H |
OH
/ H - C = 0 |
H - Cl | H - C ≡ N |
Answer:
CH4: A very symmetrical molecule (a so called tatraedric shape) without any polarity in the bondings.
There ar no free electron pairs. And there is no tendency to donate or to capture.
H2S: A small difference in electronegativity; there are two free electron pairs.
There will be a weak tendency to donate H+; H2S is therefore a very weak acid.
Furtheron we will see the link between the position of S in the Periodic Table and the acidness of this substance.
NH3: Has clearly some more difference in electronegativity; and one free electron pair.
In this molecule dominates the tendency to pick up a proton. This is a weak base.
H2O: The division of electrons is equal to that of H2S, but there is a bigger difference in electronegativity. The watermolecule can donate as it can capture (so it is amphoteric); but as an acid and as a base, water is very weak.
HCOOH: The O - H bond in the carboxyl group has a solid polarity; the O has two free electron pairs, de H can more or less be donated. The substance is an acid (HCOOH is one of the strongest acids in the carbon chemistry!)
HCl: Very stong acid. The bonding between H and Cl is very polar and very ready to donate H+-ions.
HCN: Little polarity between H and C, but the presence of N causes a small tendency to donate protons. So this is a weak acid.
Q 09-12
Does this make sense?: Cationic acids are - in general - solutions with very positive metal ions (mostly 3+).
Answer:
Those positive ions contain a 3+ charge (some have 2+ of 4+). In aqueous solutions, these ions are heavily surrounded by water molecules with there Hδ+-atomen.
The more positive central charge, the more repulsive power there will be by those ions on the Hδ+ of the water molecule.
Completely in accordance with this phenomena, this complex will be capable to donate H+-ions, wether difficult or easily.
Q 09-16
Give an organic substance, also being a diprotonic base.
Answer:
A nice example is the ion 'oxalate': C2O42-
This ion can take up two protons and is thus a twoprotonic base in the carbon chemistry.
Q 09-24
- HA + H 2O
 H3O+ + A-
H3O+ + A-
- A- + H2O
 HA + OH-
HA + OH-
Which ones?
Answer:
The conjugated pairs are:
- HA en A-
- H2O and H3O+
- H2O and OH-
Q 09-27
Explain if the following salts have the possibility to donate or pick up protons:
sodium carbonate and calcium hydrogen carbonate.
Answer:
Na2CO3 is built up of the ions Na+ and CO32-. If you dissolve this salt in water, all ions come free; the substance is very well soluble.
Na+ does nothing with water and does not form any acid of base.
However, CO32- is an ion that reacts with water; it picks up H+ ions from water molecules. Than remains OH- that is capable to pick up H+. Any solution with these carbonate ions will react as a base.
A solution of sodium carbonate (=soda) will react basic and will have a pH value above 7.
Calcium hydrogen carbonate has the formula Ca(HCO3)2 and will dissolve in water (all hydrogen salts are soluble). In the meantime ions of Ca2+ and HCO3- are produced.
The Calcium ions have practically no influence on the pH of a solution; it is hardly acid or basic.
However, HCO3- is another story: This ion is amphoteric; can take up protons (and thus form H2CO3) and can donate protons (and form CO32-).
If an ion can react acid as well as basic, the question is which tendency will dominate: the acid of the basic. Investigate the acid constant and teh base constant of HCO3-, because the biggest wins.
In tabel I you will find the values: KA = 10-10 and KB= 10-8.
The base constant is 100 x bigger, or: as a base the ion is stronger than as an acid. This ion in water will therefore cause a (weak) basic environment with a ph over 7.
Q 09-33
A certain solution in water shows different colours with indicators:
Litmus becomes blue; Bromiumthymolblue becomes blue and fenolftaleine becomes colourless.
Which are the bounderies in between which the pH of this solution remains? Explain your answer.
Answer:
| indicator | acid colour | basic colour |
| Litmus | red until pH 5,5 | blue from pH 8,0 |
| Bromiumthymolblue | red until pH 1,2 |
yellow from pH 2,8 till 6,0 and blue from 7,6 |
| Fenolftaleine | colourless until pH 8,2 | violet from pH 10 |
- Litmus is bluw, so the pH must be bigger than 8.0
- Bromiumthymolblue is blue too, so the pH must be bigger than 7,6
- Fenolftaleine is colourless, so the pH must be smaller than 8,2
Q 09-38
- You have 500 ml 0.1M NaAc-solution (sodium acetate) of 25 degrees Celcius. Adding 2 ml 2M HCl decreases immediatly the pH from 8,87 to 3,68
- The same addition to 500 ml buffer solution (of 0.1MHAc and 0.1M NaAc) decreases the pH only from 4,74 to 4,68.
- Adding not acid, but 2 ml 2M NaOH to the same buffersolution gives a very small rise of pH.
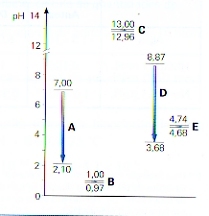
In this figure you can see such changes: five times, 2 ml 2M HCl is added to 500 ml ...
- pure water
- a solution of 0,1M strong acid
- a solution of 0,1M strong base
- a solution of 0,1M weak base
- a buffer solution
- check the affirmations of the above I and II through calculations with the bufferformula.
- calculate the pH change after the above adding III, of 2 ml 2M NaOH to 500 ml buffer 0.1M NaAc/HAc
- Check and explain the data in the diagram.
a.
You have 500 ml 0.1M NaAc-solution (sodium acetate) of 25 degrees Celcius. Je hebt 500 ml oplossing van 25 graden Celcius van 0,1M NaAc (natriumacetaat). Adding 2 ml 2M HCl decreases immediatly the pH from 8,87 to 3,68 You have 2 ml 2M HCl. That means: HCl has a concentration of 2 mol/l = 2·10-3mol HCl/ml and so 4·10-3mol HCl/2 ml
In other words: you add 4·10-3mol HCl to 500 ml 0,1M HaAc
0,1M HAc means: 10-1mol HAc/l = 0,5·10-1mol HAc/500 ml
So you join 4·10-3mol HCl with 0,5·10-1mol HAc
HCl is strong and is completely dissociated in ions, so there are 4·10-3mol H+ in 502 ml
HAc is weak, and the equilibrium HAc
The concentration [H+] (or [H3O+] is 4·10-3mol H+ / 502 ml = 1000/502 · 4·10-3mol H+ / l = 0.002008 mol/l → pH = 3-log2.008 = 3-0.303 = 2.67
b.
The same addition to 500 ml buffer solution of 0,1M HAc + 0,1M NaAc lowers the pH only from 4,74 to 4,68, being only a decrease of 0.06
So you add 2·10-3mol HCl, but this time to a buffer solution.
The buffersolution before the adding has a pH that you can calculate with the buffer formula:
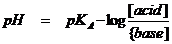
The value of pKA of the buffer 'acetic acid-acetate' you can find in tabel I {=±4. In other tables you can find more exact values. Just let us calculate with 4. That is less exact, but we don't care. We fill in de value of 4 in the buffer formula.
The concentrations of the acid HAc and of the base Ac- both are: 0.1 Those values we fill in in the buffer formula. Answer: pH = 4
in the above affirmation, the answer must be 4.74, zo there they applied more exact values of the acid constant. Thats why.
If we add 2·10-3mol H+, than this reacts immediatly with the same amount of the weak base Ac- while a similar amount of weak acid HAc is formed extra.
The concentration become:
[HAc] = 0.05 + 0.002 mol/502 ml = ±0.104 mol/l
[Ac-] = 0.05 - 0,002 mol/502 ml = ±0.096 mol/l
According to the bufferformula, now pH = pKA - log[acid]/[base] = 4 - log [0.104 / 0.096] = 4 - log 1.083 = 4 - 0.035 = 3.965
you see: pH decreases with 0.035
The given decrease from 4.74 naar 4.68, is 0,06; also very small, but our calculation is based on a rough pH estimation of 4. You can see that the influence on the pH by this adding is very small.
c.
Adding to the same buffer solution, not acid, but 2 ml 2M NaOH, than the pH will raise only a very littlebit. Adding 2·10-3mol OH-, this will immediatly react with a similar amount of weak acid HAc and the same amount of weak base Ac- is formed extra.
The new concentration become:
[HAc] = 0.05 - 0.002 mol/502 ml = ±0.096 mol/l
[Ac-] = 0,05 + 0,002 mol/502 ml = ±0,104 mol/l
According to the buffer formula we get now: pH = pKA - log[acid]/[base] = 4 - log [0.096 / 0.104] = 4 - log 0.92 = 4 - -0.035 = 4.035
The pH raises with 0.035
Q 09-42
Making compounds with Hydrogen, Carbon and Silicon of group 4 do not participate in the formation of acids and bases. What could be the reason for that?
Answer:
The compounds are CH4 and SiH4. The difference in electronegativity is very small in these molecules
Q 09-44
Is the following mixture a neutral solution?: 1 mol H2SO4 + 1 mol NaOH in water
Explain your answer.
Answer:
At joining 1 mol sulfuric acid and 1 mol sodiumhydroxyde, the two will react immediatly (strong acid with strong base) according to the reaction:
H2SO4 + 2NaOH
As you can see, the mol proportion is 1 : 2. This means that you will need twice as much base as acid for a full and complete reaction. Still of both only 1 mol is used. So: there is not enough base. Only half of the sulfuruc acid will be neutralised. The other half remains and creates an acid final solution.
Q 09-52
At a titration of 10 ml xM HNO3 were needed 19.87 ml 0.0978M NaOH.
The molarity of the HNO3-solution is close to 0.2M, but we don't know exactly.
Calculate the exact molarity.
Answer:
In some books they apply here a mathematical formula: n=cV.
I don't like this approach!. Better avoid automatic solutions by simply filling in forlulas etc. Yes, you get an answer and maybe that answer is right, but do you learn if understanding is not there?
Much better is a good idea of what really happens at such a titration and, based on that image, execute a clear calculation.
So study well the following solutions of the problem:
A titration always is based on a chemical reaction that occurs during that titration in the titration vessel.
In this case, at opening of the tap the added KOH(aq) immediatly will start to react with HNO3(aq).
During the process products are formed: KNO3(aq) + water.
So:
- The reaction equation: KOH(aq) + HNO3(aq) → KNO3(aq) + H2O(l)
- The MOL-proportion of the reactant = 1 : 1 (1 mol KOH reacts with 1 mol HNO3)
- We want to calculate the number of mol HNO3
- We added from the burette 0.0978M NaOH toe, i.e. 0.0978 mol NaOH per liter = 0,0978 mmol per ml.
adding of one ml of the KOH means: adding of 0.0978 millimol (mmol) KOH.
In reality we added 19.87 ml KOH and this contains 1987 x 0.0978 mmol KOH = 1.9433 mmol KOH →
during the titration reacted (mol proportion 1:1) 1.9433 mmol KOH with 1.9433 mmol HNO3
These 1.9433 mmol HNO3 originally were present in the 10 ml of the nitric acid solution.
→ the concentration of the nitric acid solution must be 1.9433 mmol/10ml
= 1.9433 x 10-3 mol/10 ml
= 1.9433 x 10-3 x 100 mol/l
= 0.1943 mol/l
Conclusion: the titrated HNO3-solution has a molarity of0.1943M
Q 09-58
Fenolftaleine and Methyl orange are acid-base-indicators. Both are weak acids, but one is less weak than the other.
Which one is the weakest? Explain your answer.
Answer:
Lets have a look at the table with indicators.
methyl oranje red 3,1 - 4,4 orange-yellow
fenolftaeine colourless 8,2 - 10 carmin red
methyl orange has a change zone at about 3 - 4 and fenolftaleine has a change zone at about 8 - 10
What does this mean for the strenghth of the indicator:
Indicators themselves are weak acids (HIn) being able to donate protons. The equilibrium is: HIn
Methyl orange has (in solution) a much lower pH than fenolftaleine and therefore we may sey that methyl orange will donate H+ more easy,i.e. will be a stronger acid, or better: less weak.
Q 10-02
How many electrons are present in the following particles:
Ba2+ SO42- 6C6H12O6 U ?
Answer:
A neutral atom of Barium has 56 electrons (in the orbitals) and the ion has two electrons less;
so: Ba2+ has 56 – 2 = 54 electrons.
The sulphate ion has one S- and four O-atoms, so 1x16 + 4x8 = 48 electrons.
The charge of the ion is 2-, so there are two extra electrons = 50 total.
6 glucose molecules have:
36 atoms C + 72 atoms H + 36 atoms O = 6 molecules of glucose: 36 atoms of C + 72 atoms of H + 36 atoms of O =
36x6 + 72x1 + 36x8 = 576 electronen in total.
1 atom Uranium has 92 electronen (=atom number).
Q 10-12
Oxidators en Reductors exist as atom, as molecule or as (complex) ion.
Choose from table X an example for every kind (so three different oxidators and three different reductors, and show the half reactions.
Answer
-
PbO2 + SO42- + 4H + + 2e-
 PbSO4 + 2H2O
PbSO4 + 2H2O
[reaction of a molecule (oxidator)] -
Cr2O72- + 14H + + 6e-
 2Cr3+ + 7H2O
2Cr3+ + 7H2O
[reaction of a complex ion (oxidator)] -
S + 2H+ + 2e-
 H2S
H2S
[reaction of an atom S (oxidator)] -
S + 2e-
 S2-
S2-
[reaction of a simple ion (reductor)] -
Zn2+ + 2e-
 Zn
Zn
[reaction of an atom (reductor)]
Q 10-18
If Iron is rusting, is this a direct redox reaction? yes or no?. Explain your answer.
Answer:
The Iron reacts directly in contact with Oxygen, even if this reaction is a slow one.
Q 10-30
- Explain why metals and graphite conduct electricity
- Explain why copper- or iron electrodes react as reductors
- Give an example of a molten substance that can conduct electricity
- Explain if destilled water can conduct electricity
Answer:
-
Metals are composed of metal lattices, and these lattices have free electrons with free movebility in the metal matter. This creates the electric conductability.
Graphite is very special matter, a form of Carbon where each Carbon atom has three covalent bonds and one 'unused' electron per atom. That electrond is fee, can move freely in the lattic of graphite, comparable with the electrons in a metal lattice.
Free charges cause always electric conductability. - Electrodes of metal have metal atoms that all have few electrons in the outside orbital. They want to donate electrons to achieve a stable electronic structure. Particles that donate electrons are reductors, according to the definition.
- Kitchen salt, Na Cl, melts at a temperature above 800şC. In the molten salt you have free ions that can move freely, so: yes, it conducts.
-
The concentration of H+ and of OH- is 2x10-7mol/l; that seems a lot, but in reality it is very few for real conduction. Distilled water has few ions, so it will hardly conduct.
Q 10-46
Which ones are the oxidators and reductors in the following reactions (check this with the oxidation numbers):
-
SO2 + Br2 + 2H2O
 2HBr + H2SO4
2HBr + H2SO4
-
Mg + H2SO4
 MgSO4 + H2
MgSO4 + H2
-
Cu + 2H2SO4
 CuSO4 + SO2 + 2H2O
CuSO4 + SO2 + 2H2O
-
3I2 + 6KOH
 KIO3 + 5KI + 3H2O
KIO3 + 5KI + 3H2O
-
S changed from +4 to +6
 donated 2 electrons per atom,
donated 2 electrons per atom,
 is reductor
Br changed from 0 to -1
is reductor
Br changed from 0 to -1  captured 1 electron op per atom,
captured 1 electron op per atom,
 is oxidator
is oxidator
-
Mg changed from 0 to +2
 donated 2 electrons per atom,
donated 2 electrons per atom,
 is reductor
H+ captured 1 electron per atom
is reductor
H+ captured 1 electron per atom  is oxidator
is oxidator
-
Cu changed from 0 to +2
 lost 2 electrons per atom,
lost 2 electrons per atom,
 is reductor
S changed from +6 to +4
is reductor
S changed from +6 to +4  took 2 electrons,
took 2 electrons,
 is oxidator
is oxidator
-
I changed from 0 to +1 (in KIO)
 lost 1 electron,
lost 1 electron,
 is reductor
I changed from 0 to -1
is reductor
I changed from 0 to -1  took 1 electron,
took 1 electron,
 I is oxidator too.
I is oxidator too.
Q 11-04
What is meant with: "thermo catalysis"?
Answer
This is a simple combination of two reaction conditions:
- Elevated temperature
- Presence of a catalyst
Q 11-08
What can we observer during that reaction with Bromine water?
Answer:
- Bromine water has a polar and aquanous environment; the color is brownish-yellow.
- ethyne is a non-polair gas.
So the Bromine will disappear during the contact with ethyne gas. Bit by bit the brownish yellow colour of the Bromine will desappear until the solution is colourless.
Q 11-10
Calciumcarbide (C2H2) is a white and solid substance with a special smell, very unstable, that spontaneously reacts with water.
Sometimes it is called: "Carbit".
The products of this reaction with water are: a gas with a sharp smell and a basic solution. If the gas is guided through Bromine water (a diluted solution of Bromine in water), the yellow colour slowly disappears.
Will there be a redox reaction in this process? Explain.
Answer:
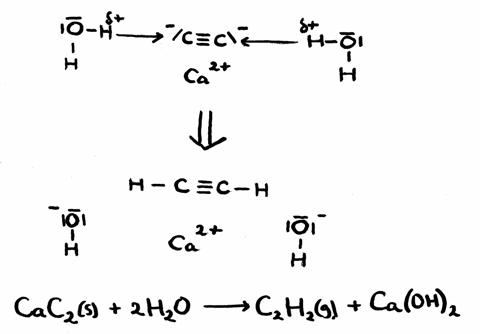
We see the H+-transfer, indicating an acid base reaction.
There is no change in oxidation number, so: this is no redox reaction.
Q 11-12
Propanol can serve as reactant in the production of propene, in an elimination-reaction.
- Give this reaction in structures
- Which substances can be helpfull in this process?
-
The propanol must lose an OH-group and a H-atom of a neighbouring C.
Thus, a water molecule is formed + an extra bonding between the two C-atoms. This created propene.
In structurs:
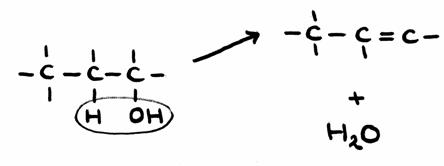
-
Looking at the necessaty to remove the water molecules, hycroscopic substances can help this reaction.
/ol>
Q 11-20
Give in chemical structures the reaction of the substitution of chlorine at toluene. And give also the conditions for that reaction.
Answer:
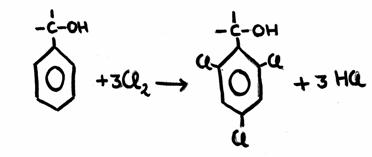
Q 11-21
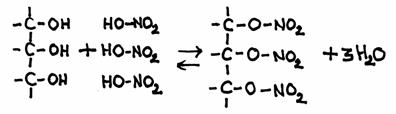
The product has the official name: glycerol-trinitrate. But in practice you can often hear another name: nitro glycerine- Give the reaction equation in molecular formulas
- Explain that the last name 'nitroglycerine' is wrong according to the UIPAC rules.
-
C3H5(OH)3 + 3HNO3
 C3H5(NO3)3
C3H5(NO3)3
-
The 'nitro' group is always directly connected to a C-chain and not via an O-atom.
In fact, here we have a tri-ester of glycerol and nitric acid, and the product contains 3 nitrate groups (= NO3-).
Q 11-26
Calculate the oxydation number of the C-atoms in the following substances (fill out in the table)
Answer:Ethane Both C-atoms are -3 ethanol one C-atom is -3 and the other is -1 ethanal one C-atom is -3 and the other is +1 ethoxy-ethane Two C-atoms are -3 and two others are -1 ethyl ethan(o)ate Two C-atoms are -3, one is +1 and one is +3 methane The C-atom is -4
Q 11-38
Affirmations:- Methanoic acid can easily be oxydised
- Ethanoic acid can easily be oxydised
Answer:- Methanoic acid = formic acid has a polar C-atom still remaining with an H-atom, so: yes, it can still be oxydised.
- The products, by the way, are Carbondioxyde and water!!
- Ethanoic acid has a polar C-atom, but no H-atom anymore, so cannot easily be oxydised.
Q 11-53
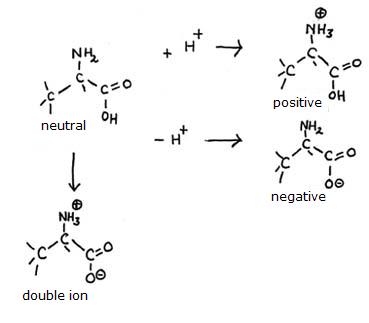
A very simple amino acid is Alanine (2-amino-propanoic acid)- Give the structure and explain where and how the amphoteric reactions take place.
- What is ment with a 'double-ion'?
-
-
In the case of external pronon transfer (with other acids or bases):
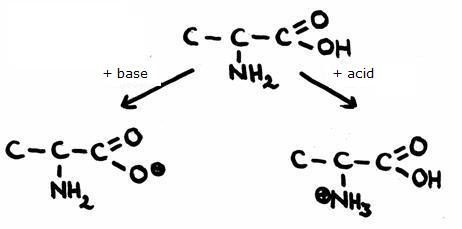
-
In the case of internal proton transfer (without any onther acids or bases):
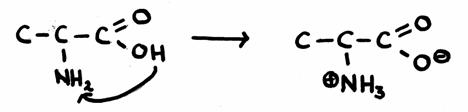
-
In the case of external pronon transfer (with other acids or bases):
- In the lower image, at the right side, you see a double ion: a structure that has a postive as well as a negative charge.
Q 12-06
Find the structure of Lysina and explain the pH of a solution of Lysina in water.
Answer:
The structure: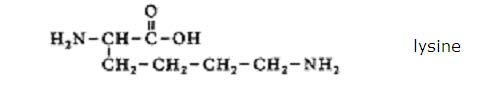
A solution of Lysina will have a pH of about 9,5, so a bit basic.
This value is due to the presence of two amino groups. Lysina has one acid group and two basic groups.
The basic character dominates pH > 7
pH > 7
Q 12-10
Demonstrate, with structures, the four configuration Alanina can have (with and wihtout charges).
Answer:
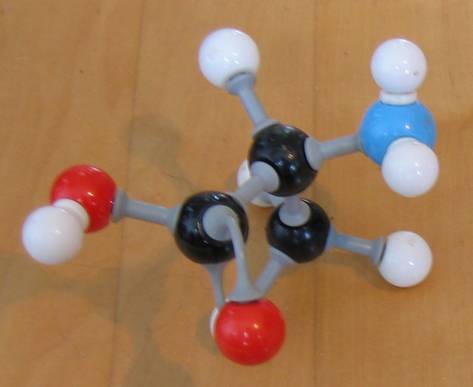
Q 12-13
Imagine a protein with 1000 units of Valine (so the monomer Valine is present 1000 times)
Calculate the molecular mass.
Answer:
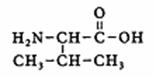
molecular mass = 5xC + 2xO + 1xN + 11xH = 117
1000 x 117 = 117,000 (the full Valine molecules)
1000 x 18 – 18,000 (the water, that is formed and is separated)
molecular mass of this protein: 117,000 – 18,000 = 99,000
Q 12-22
True or False:
To investigate the presence of glucose in urine, a more specific method than with Fehlings Reagent is the enzymatic method with glucose oxidase.
Answer:
This is true. Enzimes - here oxidase - are many more times specific than common chemicals. Fehlings Reagent reacts with many more weak reductors than just glucose.
Q 12-24
Imagine that somewhere a human being has succeeded (i.g. by mutation of its genes) to acquire the possibility to realise photosynhtesis somewhere in its body.
What could be the consequence?
Answer:
This human being could have a green colour, probably the skin (so marsmen have clorophyl in their skin!). En they have the option, under influence of the sun, to produce sugar out of carbondioxyde and water.
He can produce his own glucose, and so consumption of starch has becoma fully unnecessary.
Q 12-33
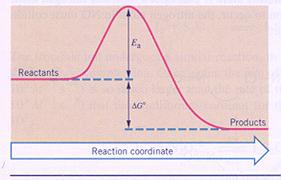
Show in an energy diagram what is the 'activating energy' of a chemical reaction.
Answer
:
Q 12-38
In the human digestion system, different enzimes are working. Each of them has its specific pH-optimum (the optimal pH value where the enzime works the best).place enzimes pH-optimum In saliva amilase and maltase 6,6 In the stomach peptase, rennase 1,5 - 4 In the pancreas amilase, maltase, lipase, tryptase, polipeptidase 6,6 - 9 In the bowels maltase, saccharase, lactase, ereptase 6,6 – 8,5 - Explain the variations in pH-values in the four places
- Why does maltase only appears at the end of the digestion system?
Answer:
- In the stomach is found a rather strong acid. In the mouth we would not bear such a low pH. In the pancreas and in the bowels the pH-value may vary a bit, dependent of the substances to be digested.
- Maltose - in general - is a product of stach hydrolysis. It cost time to cut stach into small pieces.
Q 12-40
Explain how you can get this expression, starting with presupposition 1.
Answer:
Presupposition 1:
A "steady state", a stationair phase in which the concentration of intermediate ES does not change: the formation of the ES complex occurs with the same rate as the disappearance (degradation) of it.
this means:
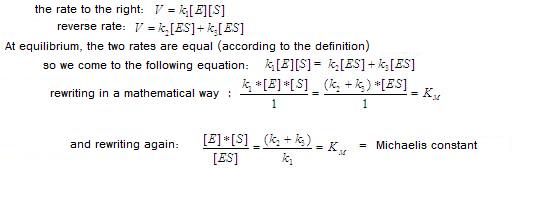
Q
Q
Q
Q
Q
Still under construction
Q
Q