|
Earth, our globe, is the living of all living creatures we know, including man, you for example.
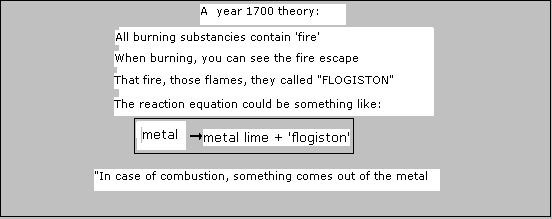
Do not neglect your own house! Take care of a good maintenance; your existence depends on it. We, men and women have the capacities and responsibilities to do so; we are no reasonless animals; we have got brains. Common sense too! And that sense should be based on knowledge, also knowledge about nature. There is nothing in small stupid talk. In this module you can collect some basic knowledge of the substances in our environment. We will treat only some substances in your environment and some of their properties, including substances that should not be there. In another mudule we will talk about all kind of reactions that take place in our environment. It is nice to include the old Greek division of substances on earth. They thought that all substances were composed of four elements: earth (solid), water (liquid), air (gas) and fire (spirit). Enything solid, hard, contains lots of 'earth'. Anything liquid must contain lots of water. anything gaseous / thinny must contain air. And yes, if you set fire to one or another substance, fire comes out! You can notice that. So fuel must contain lots of fire. Funny yes, but modern science has complete different ideas. |
| ELEMENTS | PERCENTAGES |
| Oxygen | 47 |
| Silicium | 28 |
| Aluminium | 8 |
| Iron | 5 |
| Calcium | 3,5 |
| Sodium | 3 |
| Potassium | 2,5 |
| Magnesium | 2 |
| Other elements | 1 |
|
ELEMENT
|
AMOUNT
mg/l |
| Chlorine | 19,2 |
| Sodium ions | 10,7 |
| Magnesium ions | 1,3 |
| Sulfite-, Potassium-, Calcium- ans Bromide-ions | ±1,8 |
| Other elements | ±1,4 |
| TOTAL: | ±35 mg/liter |
| SUBSTANCE | PERCENTAGE |
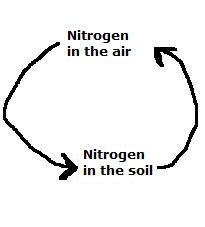
| Nitrogen | 78 |
| Oxygen | 21 |
| Argon | 1 |
| Carbon dioxyde | 0,04 (!) |
| other noble gases | 0,003 |