| ZUUR | Ka | pKa | BASE | Kb | pKb | |
| HClO4 | >>>1 | <<0 | ClO4- | <<<1 | >>14 | |
| HI | >>>1 | <<0 | I- | <<<1 | >>14 | |
| HBr | >>>1 | <<0 | Br- | <<<1 | >>14 | |
| HCl | >>>1 | <<0 | Cl- | <<<1 | >>14 | |
| H2SO4 | >>>1 | <<0 | HSO4- | <<<1 | >>14 | |
| HNO3 | >>>1 | <<0 | NO3- | <<<1 | >>14 | |
| HClO3 | >>>1 | <<0 | ClO3- | <<<1 | >>14 | |
| H3O+ | ±55.6 | ±1.75 | H2O | ±0.02 x 10-14 | ±15.75 | |
| (H2SO3) | 10-2 | ±2 | HSO3- | ±10-12 | ±12 | |
| HSO4- | 10-2 | ±2 | SO42- | ±10-12 | ±12 | |
| H3PO4 | 10-2 | ±2 | H2PO4- | ±10-12 | ±12 | |
| HF | 10-3 | ±3 | F- | ±10-11 | ±11 | |
| HCOOH | 10-4 | ±4 | HCOO- | ±10-10 | ±10 | |
| CH3COOH | 10-4 | ±4 | CH3COO- | ±10-10 | ±10 | |
| Al(H2O)63+ | 10-5 | ±5 | Al(OH)(H2O)52+ | ±10-9 | ±9 | |
| (H2CO3) | 10-6 | ±6 | HCO3- | ±10-8 | ±8 | |
| H2S | 10-7 | ±7 | HS- | ±10-7 | ±7 | |
| H2PO4- | 10-7 | ±7 | HPO42- | ±10-7 | ±7 | |
| HSO3- | 10-8 | ±8 | SO32- | ±10-6 | ±6 | |
| NH4+ | 10-10 | ±10 | NH3 | ±10-4 | ±4 | |
| HCO3- | 10-10 | ±10 | CO32- | ±10-4 | ±4 | |
| HPO42- | 10-13 | ±13 | PO43- | ±10-1 | ±1 | |
| HS- | 10-13 | ±13 | S2- | ±10-1 | ±1 | |
| H2O | ±0.02 x 10-14 | ±15.75 | OH- | ±55,6 | ±1.75 | |
| hoe lager in deze tabel, | hoe zwakker het zuur | hoe lager in deze tabel, | hoe sterker de base |
A a |
alfa | B b |
beta | Dd |
delta | E e |
epsilon | |||
| G g |
gama | C c |
chis | F j |
fi | H h |
eta | |||
| I i |
iota | K k |
kapa | L l |
lambda | M m |
mi | |||
| N n |
ni | O o |
omicron | P p |
pi | Q q |
teta | |||
| R r |
rho | S s |
sigma | T t |
tau | U u |
upsilon | |||
| W w |
omega | X x |
csi | Y y |
psi | Z z |
zeta |
| Water: | H2O | ± 60% |
| Ander materiaal: | H | 3% |
| C | 20 % | |
| O | ± 9 % | |
| N | ± 3 % | |
| Ca | ± 2,5 % | |
| P | ± 1,1 % | |
| Overige elementen | ± <1 % |
H-H |
0,74 | H-F |
0,92 | N≡N |
1,10 | C-F |
1,38 |
F-F |
1,42 | H-Cl |
1,27 | C≡C |
1,21 | C-Cl |
1,77 |
Cl-Cl |
1,99 | H-Br |
1,41 | C≡N |
1,16 | C-Br |
1,94 |
Br-Br |
2,28 | H-I |
1,60 | C=O (CO2) |
1,16 | C-I |
2,14 |
I-I |
2,67 | H-O |
0,96 | C=O |
1,22 | C-O |
1,43 |
N-N |
1,47 | N-H |
1,01 | C=C |
1,35 | H-S |
1,34 |
C-C |
1,54 | C-H |
1,08 | O=O |
1,21 | P-H |
1,42 |
| 1 = elementen |
2 = symbool |
3 = atoom- nummer |
4 = atoom- massa |
5 = electro negati viteit |
6 = smeltpunt |
7 = kookpunt |
8 = atoom- straal x 10-12m |
9 = ion- straal x 10-12m |
10 = v-d-Waals straal x 10-12m |
| Actinium | Ac | 89 | 1,1 | 1324 | 3500 | 188 | |||
| Aluminium | Al | 13 | 27 | 1,6 | 933 | 2792 | 143 | 45 | |
| Amerikanum | Am | 95 | 1,3 | 1400 | 2900 | 173 | |||
| Antimoon | Sb | 51 | 121,8 | 2,1 | 904 | 1860 | 141 | 245 | 220 |
| Argon | Ar | 18 | 39,9 | 84 | 87 | 192 | |||
| Arseen | As | 33 | 74,9 | 2,2 | 1090 | 887 | 121 | 222 | 200 |
| Astatium | At | 85 | 2,2 | 575 | 610 | 140 | |||
| Barium | Ba | 56 | 137,3 | 0,9 | 1000 | 2170 | 217 | 134 | |
| Berilium | Be | 4 | 9,0 | 1,6 | 1560 | 2744 | 112 | 30 | |
| Berkelium | Bk | 97 | 1,3 | 1300 | 2900 | 172 | |||
| Bismuth | Bi | 83 | 209,0 | 2,0 | 545 | 1837 | 170 | ||
| Boor/Borium | B | 5 | 10,8 | 2,0 | 2348 | 4273 | 88 | 16 | 217 |
| Broom | Br | 35 | 79,9 | 3,0 | 266 | 332 | 114 | 196 | 195 |
| Cadmium | Cd | 48 | 112,4 | 1,7 | 594 | 1040 | 149 | 97 | |
| Calcium | Ca | 20 | 40,1 | 1,0 | 1115 | 1757 | 197 | 94 | |
| Californium | Cf | 98 | 1,3 | 1200 | 1700 | 199 | |||
| Koolstof | C | 6 | 12,0 | 2,6 | 3823 | 4098 | 77 | 185 | |
| Cerium | Ce | 58 | 140,1 | 1,1 | 1070 | 3698 | 183 | 101 | |
| Cesium | Cs | 55 | 132,9 | 0,9 | 302 | 944 | 262 | 167 | |
| Lood | Pb | 82 | 207,5 | 1,6 | 601 | 2022 | 175 | 120 | |
| Chloor | Cl | 17 | 35,5 | 3,2 | 172 | 239 | 99 | 181 | 180 |
| Cobalt | Co | 27 | 58,9 | 1,7 | 1968 | 3200 | 125 | 74 | |
| Koper | Cu | 29 | 63,5 | 1,8 | 1357 | 2835 | |||
| Crypton | Kr | 36 | 83,8 | 116 | 120 | 197 | |||
| Chroom | Cr | 24 | 52,0 | 1,6 | 2180 | 2944 | 125 | 63 | |
| Curium | Cm | 96 | 1600 | 3400 | 174 | ||||
| Dysprosium | Dy | 66 | 162,5 | 1,2 | 1681 | 2835 | 175 | ||
| Einsteinium | Es | 99 | 1100 | 1800 | 203 | ||||
| Zwavel | S | 16 | 32,1 | 2,5 | 388 | 718 | 104 | 190 | 185 |
| Erbium | Er | 68 | 167,3 | 1802 | 3136 | 173 | |||
| Scandium | Sc | 21 | 45,0 | 1,2 | 1814 | 3195 | 160 | ||
| Tin (Stannium) | Sn | 50 | 118,7 | 1,7 | 505 | 2875 | 162 | 112 | |
| Strontium | Sr | 38 | 87,6 | 1,0 | 1050 | 1655 | 215 | 110 | |
| Europium | Eu | 63 | 152,0 | 1099 | 1875 | 204 | |||
| IJzer (Ferrum) | Fe | 26 | 55,8 | 1,6 | 1811 | 3134 | 126 | 76 | |
| Fluor | F | 9 | 19,0 | 4,0 | 54 | 85 | 64 | 133 | 135 |
| Fosfor | P | 15 | 31,0 | 2,1 | 317 | 550 | 110 | 212 | 190 |
| Francium | Fr | 87 | 0,9 | 300 | 950 | 270 | 180 | ||
| Gadolinium | Gd | 64 | 157,3 | 1,1 | 1585 | 3534 | 179 | ||
| Gallium | Ga | 31 | 69,7 | 1,8 | 303 | 2477 | 141 | ||
| Germanium | Ge | 32 | 72,6 | 2,0 | 1211 | 3106 | 122 | 202 | |
| Hafnium | Hf | 72 | 178,5 | 1,2 | 2506 | 4876 | 144 | ||
| Helium | He | 2 | 4,0 | 2 | 4 | 157 | 99 | ||
| Waterstof | H | 1 | 1,0 | 2,2 | 14 | 20 | 30 | 120 | |
| Holmium | Ho | 67 | 164,9 | 1,2 | 1738 | 2968 | 174 | ||
| Indium | In | 49 | 114,8 | 1,5 | 430 | 2345 | 166 | ||
| Jodium | I | 53 | 126,9 | 2,7 | 387 | 458 | 133 | 219 | 215 |
| Iridium | Ir | 77 | 192,2 | 1,6 | 2719 | 4701 | 135 | ||
| Yterbium | Yb | 70 | 173,0 | 1,1 | 1097 | 1467 | 194 | ||
| Ytrium | Y | 39 | 88,9 | 1,1 | 1799 | 3609 | 180 | ||
| Lantanium (Lantaan) | La | 57 | 138,9 | 1,1 | 1193 | 3728 | 188 | ||
| Lithium | Li | 3 | 6,9 | 1,0 | 454 | 1615 | 152 | 68 | |
| Lutetium | Lu | 71 | 175,0 | 1,2 | 1928 | 3668 | 172 | ||
| Magnesium | Mg | 12 | 24,3 | 1,2 | 923 | 1363 | 160 | 65 | |
| Mangaan | Mn | 25 | 54,9 | 1,6 | 1915 | 2334 | 129 | 80 | |
| Kwik | Hg | 80 | 200,6 | 1,4 | 234 | 630 | 152 | 127 | |
| Molybdeen | Mo | 42 | 95,9 | 1,3 | 2856 | 4912 | 136 | ||
| Neodimium | Nd | 60 | 144,2 | 1,2 | 1293 | 3300 | 181 | ||
| Neon | Ne | 10 | 20,2 | 25 | 27 | 160 | |||
| Niobium | Nb | 41 | 92,9 | 1,2 | 2750 | 5017 | 141 | ||
| Nikkel | Ni | 28 | 58,7 | 1,8 | 1728 | 3186 | 124 | 72 | |
| Stikstof (Nitrogenium) | N | 7 | 14,0 | 3,0 | 63 | 77 | 70 | 171 | 150 |
| Osmium | Os | 76 | 190,2 | 1,5 | 1828 | 3236 | 134 | ||
| Goud (Aurum) | Au | 79 | 197,0 | 1,4 | 1337 | 3129 | 144 | 137 | |
| Zuurstof (Oxigenium) | O | 8 | 16,0 | 3,4 | 54 | 90 | 66 | 146 | 140 |
| Paladium | Pd | 46 | 106,4 | 1,4 | 1828 | 3236 | 138 | ||
| Platina | Pt | 78 | 195,1 | 1,4 | 2042 | 4098 | 138 | ||
| Plutonium | Pu | 94 | 910 | 3600 | 159 | ||||
| Polonium | Po | 84 | 1,8 | 527 | 1235 | 140 | |||
| Kalium | K | 19 | 39,1 | 0,8 | 336 | 1032 | 231 | 133 | |
| Praseodimium | Pr | 59 | 140,9 | 1,2 | 1208 | 3785 | 182 | ||
| Zilver (Argentum) | Ag | 47 | 107,9 | 1,9 | 1235 | 2435 | 144 | 126 | |
| Prometium | Pm | 61 | 1,2 | 1341 | 3003 | 183 | |||
| Proactinium | Pa | 91 | 1,5 | 1800 | 4400 | 156 | |||
| Radium | Ra | 88 | 0,9 | 972 | 1413 | 220 | 148 | ||
| Radon | Rn | 86 | 202 | 211 | |||||
| Renium | Re | 75 | 186,2 | 1,9 | 3459 | 5869 | 137 | ||
| Rhodium | Rh | 45 | 102,9 | 2,3 | 2237 | 2968 | 134 | ||
| Rubidium | Rb | 37 | 85,5 | 0,8 | 312 | 961 | 244 | 148 | |
| Rutenium | Ru | 44 | 101,1 | 2,2 | 2607 | 4423 | 133 | ||
| Samarium | Sm | 62 | 150,4 | 1,2 | 1345 | 2064 | 180 | ||
| Selenium | Se | 34 | 79,0 | 2,6 | 494 | 958 | 117 | 202 | 200 |
| Silicium | Si | 14 | 28,1 | 1,9 | 1687 | 3538 | 117 | 224 | |
| Natrium | Na | 11 | 23,0 | 0,9 | 371 | 1156 | 186 | 98 | |
| Thallium | Tl | 81 | 204,4 | 2,0 | 577 | 1746 | 171 | ||
| Tantalium | Ta | 73 | 180,9 | 1,3 | 3269 | 5731 | 143 | ||
| Technetium | Tc | 43 | 1,9 | 2430 | 4538 | 135 | |||
| Tellurium | Te | 52 | 127,6 | 2,1 | 723 | 1261 | 137 | 222 | 220 |
| Therbium | Tb | 65 | 158,9 | 1,2 | 1629 | 3396 | 167 | ||
| Titaan (Titanium) | Ti | 22 | 47,9 | 1,5 | 1941 | 3560 | 146 | 90 | |
| Thorium | Th | 90 | 232,0 | 1,3 | 2000 | 4800 | 180 | ||
| Thulium | Tm | 69 | 168,9 | 1,2 | 1818 | 2221 | 172 | ||
| Uranium | U | 92 | 238,0 | 1,4 | 1400 | 4200 | 138 | ||
| Vanadium | V | 23 | 50,9 | 1,6 | 2183 | 3680 | 131 | ||
| Wolffraam | W | 74 | 183,9 | 2,4 | 3695 | 5828 | 137 | ||
| Xenon | Xe | 54 | 131,3 | 161 | 165 | 217 | |||
| Zink | Zn | 30 | 65,4 | 1,7 | 693 | 1180 | 133 | 74 | |
| Zirkonium | Zr | 40 | 91,2 | 1,3 | 2128 | 4682 | 157 |
H-H |
- 4,36 | H-F |
- 5,63 | N≡N |
- 9,45 | C-F |
- 4,4 |
F-F |
- 1,53 | H-Cl |
- 4,32 | C≡C |
- 8,3 | C-Cl |
- 3,3 |
Cl-Cl |
- 2,43 | H-Br |
- 3,66 | C≡N |
- 8,9 | C-Br |
- 2,8 |
Br-Br |
- 1,93 | H-I |
- 2,99 | C=O |
- 8,0 | C-I |
- 2,4 |
I-I |
- 1,51 | H-O |
- 4,646 | C=S |
- 2,6 | C-O |
- 3,5 |
H-O (alcanol) |
- 4,5 | N-H |
- 3,9 | C=C |
- 6,1 | H-S |
- 3,44 |
C-C |
- 3,5 | C-H |
- 4,1 | O=O |
- 4,98 | P-H |
- 3,22 |
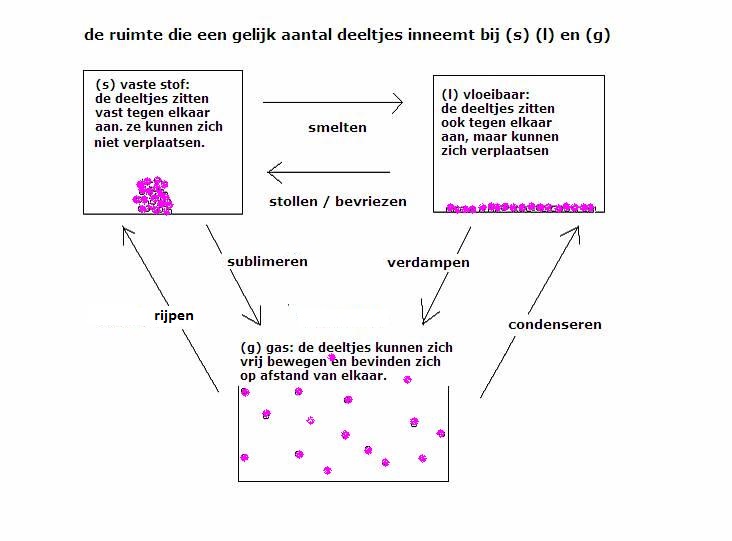
| INDICATOR | kleur bij lage pH | overgangszone | kleur bij hoge pH |
| hematoxiline | rood | 0,0 - 1,0 | geel |
| cresolrood | rood | 0,2 - 1,8 | geel |
| thymolblauw | rood | 1,2 - 2,8 | geel |
| dimethylgeel | rood | 2,9 - 4,0 | geel |
| methyloranje | rood | 3,1 - 4,4 | oranje-geel |
| methylrood | rood | 4,0 - 6,0 | geel |
| broomfenolrood | geel | 5,2 - 6,8 | violet |
| lakmoes | rood | 5,5 - 8,0 | blauw |
| broomthymolbauw | geel | 6,0 - 7,6 | blauw |
| fenolrood | geel | 6,8 - 8,4 | rood |
| thymolblauw | geel | 8,0 - 9,6 | blauw |
| fenolftaeíne | kleurloos | 8,2 - 10 | carmijnrood |
| alizarine-R-geel | geel | 10,1 - 12 | violetblauw |
| 1,3,5-trinitrobenzeen | kleurloos | 12 - 14 | oranje |
| algemene naam | algemene formule | opmerkingen |
| Alkanen | CnH2n+2 | alleen enkelvoudige C-C bindingen in de moleculen |
| Alkenen | CnH2n | dubbele C=C bindingen in de moleculen |
| Alkynen | CnH2n-2 | drievoudige C≡C in de moleculen |
| Cicloalkanen | CnH2n | |
| Benzeen | C6H6 |
| Slechts één C-atoom: | met... | zes C-atomen: | hex..... | |
| Twee C-atomen: | et..... | Zeven C-atomen: | hept...... | |
| Drie C-atomen: | prop..... | Acht C-atomen: | oct....... | |
| Vier C-atomen: | but...... | Negen C-atomen: | non...... | |
| Vijf C-atomen: | pent....... | Tien C-atomen: | dec........ |
| naam | algemene formule |
achtervoegsel | voorbeeld | kommentaar / alternatief |
| alkaan | CnH2n+2 | -aan | 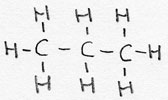
propaan |
Verzadigd |
| alkeen | CnH2n | -een | 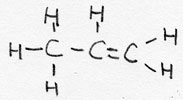 Propeen |
Onverzadigd |
| Alkyn | CnH2n-2 | -yn | 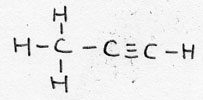
Propyn |
Onverzadigd |
| Alkyl- | CnH2n+1 -- | -yl |
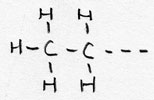
Etyl--- |
zijketen/vertakking |
| ciclo-alkaan | CnH2n | -aan |
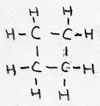
ciclo-butaan |
isomeer met alkenen/ verzadigd |
| alkanol | CnH2n+1 -OH | -ol |
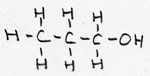
1-propanol |
voorvoegsel: hydroxi; / ook alcohol genoemd |
| alkanal | CnH2n+1-CHO | -al |
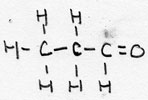
Propanal |
aldehyde |
| alkanon | CnH2n+1- -CO-CmH2m+1 |
-on |
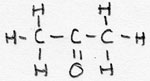
Propanon |
een ketongroep aanwezig |
| alkaanzuur | CnH2n+1-COOH |
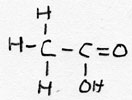
etaanzuur |
azijnzuur | |
| Alkoxi-alkaan | CnH2n+1- O-
CmH2m+1 |
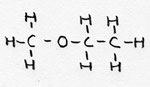
Metoxi-etaan |
Ether (2 alkylgroepen via een O verbonden) | |
| Alkyl-alkan(o)aat | CnH2n+1-COO- /
CmH2m+1 |
-yl -aat |
aat-.jpg)
Metyl-etanoaat |
een ester |
| Alkylamine (prim) | CnH2n+1-NH2 | -amine |
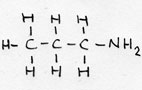
propyl-amina of: 1 aminopropaan |
voorvoegsel: amino- |
| nitro-alkaan | CnH2n+1-NO2 | 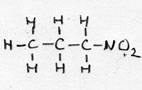
1-nitro propaan |
voorvoegsel: nitro- | |
| peptide | CnH2n+1-NH-CO-CmH2m+1 |
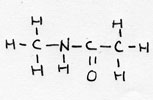 |
| oxidatoren | reductoren | ||||||||||
| F2 | + | 2e- | 2F- | ||||||||
| H2O2 | + | 2H+ | + | 2e- | 2H2O | ||||||
| PbO2 | + | SO42 - | + | 4H+ | + | 2e- | PbSO4 | + | 2H2O | ||
| MnO4- | + | 8H+ | + | 5e- | Mn2+ | + | 4H2O | ||||
| Au3+ | + | 3e- | Au | ||||||||
| Cl2 | + | 2e- | 2Cl- | ||||||||
| Cr2O72- | + | 14H+ | + | 6e- | 2Cr3+ | + | 7H2O | ||||
| MnO2 | + | 4H+ | + | 2e- | Mn2+ | + | 2H2O | ||||
| O2 | + | 4H+ | + | 4e- | 2H2O | ||||||
| Br2 | + | 2e- | 2Br- | ||||||||
| NO3- | + | 2H+ | + | e- | NO2 | + | H2O | ||||
| Ag+ | + | e- | Ag | ||||||||
| Fe3+ | + | e- | Fe2+ | ||||||||
| I2 | + | 2e- | 2I- | ||||||||
| MnO4- | + | 2H2O | + | 3e- | MnO2 | + | 4OH- | ||||
| Cu2+ | + | 2e- | Cu | ||||||||
| Cu2+ | + | e- | Cu+ | ||||||||
| S | + | 2H+ | + | 2e- | H2S | ||||||
| S4O62- | + | 2e- | 2S2O32- | + | H2O | ||||||
| 2H+ | + | 2e- | H2 | ||||||||
| SO42- | + | 2H+ | + | 2e- | SO32- | + | H2O | ||||
| Pb2+ | + | 2e- | Pb | ||||||||
| Ni2+ | + | 2e- | Ni | ||||||||
| PbSO4 | + | 2e- | Pb | + | SO42- | ||||||
| Fe2+ | + | 2e- | Fe | ||||||||
| S | + | 2e- | S2- | ||||||||
| 2 CO2 | + | 2H+ | + | 2e- | H2C2O4 | ||||||
| Zn2+ | + | 2e- | Zn | ||||||||
| 2H2O | + | 2e- | H2 | + | 2OH- | ||||||
| Al3+ | + | 3e- | Al | ||||||||
| Mg2+ | + | 2e- | Mg | ||||||||
| Al(OH)4- | + | 3e- | Al | + | 4OH- | ||||||
| Na+ | + | e- | Na | ||||||||
| Ba2+ | + | 2e- | Ba | ||||||||
| Ca2+ | + | 2e- | Ca | ||||||||
| K+ | + | e- | K | ||||||||
| Hoe lager | hoe zwakker de oxidator | Hoe lager, | hoe sterker de reductor |
| OH- | O2- | Cl- | Br- | I- | S2- | SO32- | SO42- | CO32- | PO43- | NO3- | CH3COO- | |
| Na+ | + |
R |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| K+ | + |
R |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| NH4+ | + |
+ |
+ |
D |
+ |
+ |
D |
D |
+ |
+ |
||
| Ca2+ | ± |
R |
+ |
+ |
+ |
± |
- |
± |
- |
- |
+ |
+ |
| Ba2+ | + |
R |
+ |
+ |
+ |
± |
- |
- |
- |
- |
+ |
+ |
| Ag+ | - |
- |
- |
- |
- |
- |
± |
- |
- |
+ |
± |
|
| Pb2+ | - |
- |
± |
± |
- |
- |
- |
- |
- |
- |
+ |
+ |
| Hg+ | - |
- |
- |
- |
- |
- |
- |
- |
- |
+ |
± |
|
| Hg2+ | - |
+ |
± |
- |
- |
R |
- |
- |
+ |
+ |
||
| Cu2+ | - |
- |
+ |
+ |
- |
- |
+ |
- |
- |
+ |
+ |
|
| Al3+ | - |
- |
+ |
+ |
+ |
R |
+ |
+ |
- |
+ |
+ |
|
| Fe2+ | - |
- |
+ |
+ |
+ |
- |
- |
+ |
- |
- |
+ |
+ |
| Fe3+ | - |
- |
+ |
+ |
- |
+ |
- |
+ |
+ |
|||
| Mg2+ | - |
- |
+ |
+ |
+ |
± |
- |
+ |
- |
- |
+ |
+ |
| Zn2+ | - |
- |
+ |
+ |
+ |
- |
- |
+ |
- |
- |
+ |
+ |
| +: oplosbaar | -: onoplosbaar | ±: enigzins oplosbaar | R: reageert met water | D: ontleedt in water |
| Tera | T | 1012 | 1 000 000 000 000 | |
| Giga | G | 109 | 1 000 000 000 | |
| Mega | M | 106 | 1 000 000 | |
| Kilo | k | 103 | 1 000 | |
| Hecto | h | 102 | 100 | |
| Deca | da | 101 | 10 | |
| Deci | d | 10-1 | 0,1 | |
| Centi | c | 10-2 | 0,01 | |
| Mili | m | 10-3 | 0,001 | |
| Micro | m | 10-6 | 0,000 001 | |
| Nano | n | 10-9 | 0,000 000 001 | |
| Pico | p | 10-12 | 0,000 000 000 001 |
naam |
MAC waarde |
Gevaar |
toxiciciteit 1 - 5 |
wat doen |
|||
acetileen |
etyn |
H-C≡C-H |
g |
explosief |
1 |
WF / O |
|
Aceton |
propanon |
CH3COCH3 |
l |
1780 |
ontvlambaar |
2 |
WF |
zoutzuur |
waterstofchloride |
HCl (aq) |
aq |
7 |
bijtend |
2 |
W |
zwavelzuur |
H2SO4 |
1 |
aq |
bijtend |
2 |
W |
|
alkohol |
etanol |
CH3CH2OH |
1 |
1000 |
1 |
W |
|
ammoniak |
NH3 |
g / aq |
18 |
gevaarlijke dampen |
2 |
WF |
|
Arseen + derivaten |
As3+ |
s |
0,03 |
giftig |
5 |
W |
|
benzeen |
C6H6 |
1 |
7,5 |
WF |
|||
broom |
Br2 |
l / aq |
0,7 |
kankerverwekkend verstikkend |
2 |
O |
|
ongebluste kalk |
calciumoxide |
CaO |
s |
5 |
1 |
W |
|
carbide |
calciumcarbide |
C22- / CaC2 |
s |
explosief |
2 |
zand er over |
|
Lood en derivaten |
Pb |
s |
0,15 |
giftig |
4 |
W |
|
cyanides |
CN- |
s |
5 |
giftig |
4 |
||
chloor |
Cl |
g / aq |
3 |
giftig |
4 |
WF |
|
chloroform |
trichloormetaan |
CHCl3 |
1 |
5 |
gevaarlijke dampen |
1 |
WF |
zwaveldioxide |
SO2 |
g |
5 |
3 |
WF |
||
ether |
Etoxi-etaan |
CH3CH2-O-CH2CH3 |
1 |
1200 |
explosief |
1 |
WF |
fenol |
hidroxibenzeen |
C6H5OH |
l |
19 |
bijtend |
2 |
|
fluor |
F2 |
g |
2 |
giftig |
4 |
||
formaline |
Metanal |
CH2O |
l |
1,5 |
bijtend |
1 |
W |
benzine |
Octanen |
C8H18 |
l / g |
explosief |
1 |
WF |
|
gycol |
1.2 dihidroxi-etaan |
HO-CH2-CH2-OH |
l |
26 |
hoofdpijn |
2 |
O |
Na/K hydroxide (aq) |
NaOH / KOH |
aq |
2 |
bijtend |
1 |
W |
|
jodium |
I2 |
s |
1 |
hoofdpijn |
2 |
W |
|
chlorix |
Natriumhypochloriet |
NaClO |
s / aq |
bijtend |
2 |
W |
|
kwik |
Hg / Hg2+ |
l |
0,05 |
giftig |
5 |
W (melk) |
|
metylalkohol |
Metanol |
CH3OH |
l |
260 |
giftig |
3 |
O |
koolmonoxide |
CO |
g |
29 |
giftig |
4 |
WF |
|
zilvernitraat |
AgNO3 |
s |
0,01 |
bijtend |
W |
||
stikstofoxides |
NxOy (NO2 NO3 etc) |
g |
4 |
hoofdpijn |
3 |
WF |
|
ozon |
O3 |
g |
giftig |
3 |
WF |
||
waterstofperoxide |
H2O2 |
l |
1,4 |
brand de huid/ explosief |
2 |
W |
|
Tetra |
tetraclorometaan |
CCl4 |
l |
12,6 |
giftig |
3 |

