We know lots of organic acids; the most important for this course are those with a carboxyl group (the alkanoic acids). Phenol is another kind of organic acid.
We will talk about carboxylic acids, amino acids, fatty acids and phenol, but also about bases.
More about this topic can be found in module 12: organic reactions.
Here only some questions
The carboxylic group is not a very strong acid.
Rather strong are methanoic acid and trichloromethanoic acid.
Comparing those two, the last one turns out to be stronger then the first one.
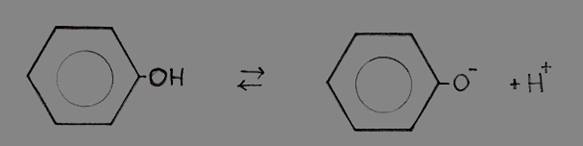
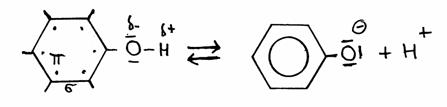
Phenol (benzene with a hydroxyl group -OH) is a liquid with a seak acid character.
Hydroxyl groups normally are found in alcohols and have no acid character at all. But the one of Phenol has, is capable to donate H+ ions from the hydroxyl group.
This remarkable fact exists thanks to the presence of that benzene ring (with the six free electrons in the ring). The absence of a proton, and so the presence of O- at the ring, will strenghten the degree of freedom of those six electrons (+1). Seven free electrons make the structure even more stable than it already was.
Losing H+ increases the stability of the structure; and that creates the (weak) acid character.
or
Phenol
The extra electron of the phenolate will participate in the free movement of the six electrons in the ring, and stabilize the phenolate.
The oxy-acids can be made - in general - from the corresponding oxyde with water.
Example: P2O5 + 3H2O
Att.: We talk now about reactions wherein the oxidation number does not change.
| Acid | Acid ion | example | example |
| hypo.......ous acid | hypo.......ite | hypochlorous acid | hypochlorite |
| ......ous acid | ......ite | chlorous acid | chlorite |
| ......ic acid | ......ate | chloric acid | chlorate |
| (hy)per.......ic acid | (hy)per.......ate | (hy)perchloric acid | (hy)perchlorate |
N.B.
Not all oxygen containing acids have all four options
But they all have the normal '...ic acid' and '....ate'
Some have also the '....ous acid' and the '....ite'
Hypo and hyper are rather exceptional
They are produced directly from an element (mostly a non metal) with Hydrogen.
The best known example is Chlorine with Hydrogen: Cl2(g)+ H2(g)
A special example is: HCN
Looking at the non metals, we come to the following Hydrogen acids: HCl, HBr, HI, H2S, HF.
Att.: there are more bondings between Hydrogen and the non metals, like H2O and NH3.
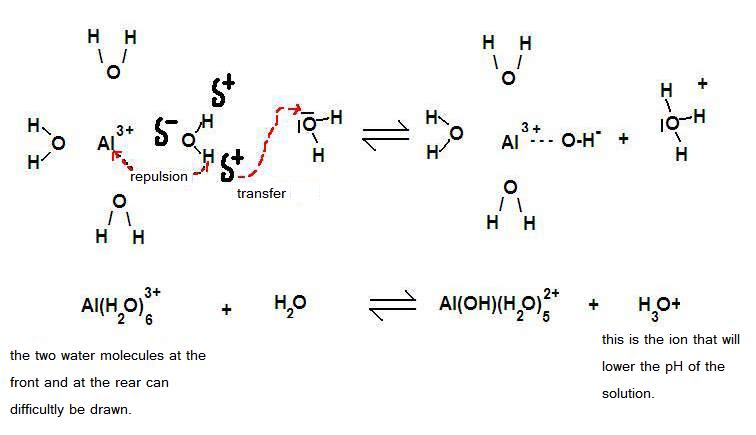
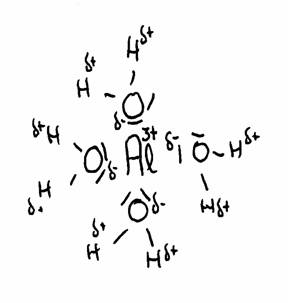
Certain polyvalent positive ions (2+ and 3+) have the property to attract water molecules. We say: they are hydrated.
There is a strong attraction to the negative charge (δ-) of those water molecules with their charge δ+ also close to those strong positive metal ions.
You must understand that this will cause repulsion: maybe protons will be repelled, donated.
cationic acids (= positive metal ion) with water
 geconjugated base + hydronium ion.
geconjugated base + hydronium ion.
Thats how appears the possibility to donate H+.
Here we deal with a number of polyvalent positive metal ions, like Al3+, Cu2+, Fe2+ or Fe3+, and more.
A special case of such a positive ion with an acid character is the ammonia ion:
NH4+ + H2O
Negative ions - normally - can be basic, or: they can catch protons.
Negative ions normally do not act as acids.
But still! There are negative ions that can act as acids, and donate H+.
Example: HCO3-.
This example shows that we deal now with amphoteric particles.
This type of negative ions still contain more Hydrogen; they come from polyprotonic acids that have not yet donated all H+.
You find themin the table with acids and bases in both colums.
Check that.