a weak organic acid
a weak organic reductor
HIn  H+ + In-
H+ + In-
RedIn  n e- + OxIn
n e- + OxIn
HIn different color than In-
RedIn different color than OxIn
the color of HIn or In-
the color of RedIn or OxIn
only can be seen when the equilibrium is sufficiently at the right or the left side.
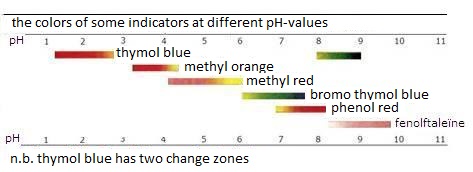
the color of an indicator depends on the environment:
In acid environment (or in a reducing environment)
the above equilibria are on the left side;
the color of HIn (or RedIn dominates.
It can be different per indicator.
In acid environment (or in a reducing environment)
the above equilibria are on the left side;
the color of HIn (or RedIn dominates.
It can be different per indicator.