Acid base reactions in Carbon Chemistry
Much information about acid base reaction can be found in module 9.
This paragraph deals with acid base reaction in the carbon chemistry.
acid + base  conjugated base + conjugated acid
conjugated base + conjugated acid
Organic acids only donate H+ coming from the hydroxy groups (-OH), in most cases part of the carboxylic structure.
In general the organic acids are weak. One of the strongest organic acids is formic acid.
Tho carboxylic group owes ist acidity to the strong polarity within that group.
It causes a strong repulsion between the C-atom and the H-atom that both have a certain positive charge (δ+).
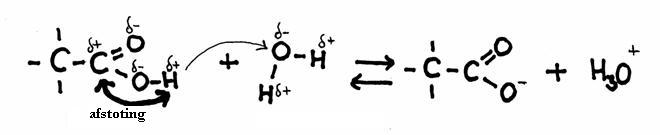
Only Hydroxy groups can lose protons, but only if the to the same C of the hydroxy group also is connected another oxygen atom, or when this hydroxygroup is connected to a benzene ring.
Mind that H atoms, directly connected to a carbon chain never perform as donator of H+
Normal alcohol groups also do not donate H+-ionen, unless very agressive substances are involved, like Na or K.
Oxalic acid (two carboxyl groups directly connected to each other = H2C2O4) is not only a stronger acid, but can also, easily, break in two parts where two molecules CO2 are formed.
If the carbon chain of an organic acid is rather lon, we call it a fatty acid.
If in that chain also are included one or more double bonds, than this molecule is a (plurally) unsaturated fatty acid.
A NH2-group, connected to a carbon chain is built up in such a way that the N-atom (more or less δ-) still has a free electron pair, available for the capturing of particles without electrons, like the H+-ion.
If that happens, the amino group becomes positive: – NH3+.
If an organic molecule (is a substance from the carbon chemistry) has an acid group as well as an amino group, than you have an amino acid.
Those two groups: carboxylic and amino, do not only react as acids or bases, but they also participate in condensation, when esters or peptides are formed.
Brought in acid environment (at low pH-values), an amino acid is positive and in basic environment (at high pH) the amino acid is negatively charged.
Somewhere between that high and low, there must be a pH value at which the amino acid molecules are neutral in average.
This special pH value is calles the the iso-electric point: het I.E.P.
att.: In the iso electric point, the amino acid molecule can occur as a so called 'double ion'.
Some complicated (aromatic) organic acids have - thanks to their structure - a certain color that changes if one or more protons are donated.
These substances are fit to act as an acid base indicator. (see also module 9)
HIn  H+ + In-
H+ + In-
color 1 color 2
