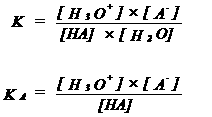
An equilibriium always will shift to the side of the weaker ones.
| HA | + | H2O | H3O+ | + | A- | |||||||||
| acid | base | acid | base | |||||||||||
| A- is conjugated base of the acid HA | ||||||||||||||
| KA = acid constant | ||||||||||||||
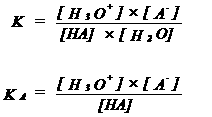
|
||||||||||||||
| If KA is very large (or pKA is very small), HA is a very strong acid | ||||||||||||||
|
An equilibriium always will shift to the side of the weaker ones. |
||||||||||||||
| A- | + | H2O | HA | + | OH- | |||||||||
| base | acid | acid | base | |||||||||||
| HA is conjugated acid of the base A- | ||||||||||||||
| KB = base constant | ||||||||||||||
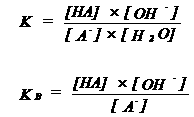
|
||||||||||||||
| If KB is very large (or pKB is very small), A- is a very strong base | ||||||||||||||
|
An equilibriium always will shift to the side of the weaker ones. |
||||||||||||||