Possess one or more carboxylis groups (-COOH) (more about this in module 11)
You must know some specials: carbonic acid, fatty acid, amino acid, phenol.
The carboxylis group can donate an ion H+, or, the carboxylic group is an acid (mostly a weak acid)
The hydroxy group normally does not donate H+, only if this one is connected to a benzene ring (phenol)
For more information about organinic acids, see the module 9 and module 11 about these topics.
They are the non-organic acids containing the element Oxygen.
Mostly can be made of, starting with the right oxyde (like: sulfur trioxyde with water --> sulfuric acid).
these acids can vary in nomenclature, depending on the minimal and maximal number of oxygen atoms in the molecule (that has to do with the so called oxydation number (see module 10). So you have H2SO3 and H2SO4
Also the conjugated basesof these acids get an own name.
example:
phosphoric acid = H3PO4. (is made of P2O5 with H2O)
If the formula is not H3PO4, but H3PO3, then this acid is called: phosphorous acid.
| Acid | Acid ion | example | example |
| hypo.......ous acid | hypo.......ite | hypochlorous acid | hypochlorite |
| ......ous acid | ......ite | chlorous acid | chlorite |
| ......ic acid | ......ate | chloric acid | chlorate |
| (hy)per.......ic acid | (hy)per.......ate | (hy)perchloric acid | (hy)perchlorate |
N.B.
Not all oxygen containing acids have all four options
But they all have the normal '...ic acid' and '....ate'
Some have also the '....ous acid' and the '....ite'
Hypo and hyper are rather exceptional
Best known of this type is HCl, hydrochloric acid
The formula starts always with H, immediately followed by another element, without Oxygen (HBr, HI, HCN, etc.)
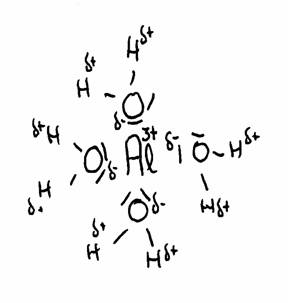
The most famous example is the Aluminium ion dissolved in water, but there are lots more. Cations normally are hydrated polypositive metal ions: Al(H2O)63+, Cu2+, Fe2+ of Fe3+, any many more, and always hydrated in water.
hydratation
The poly positive ions attract closely, in watery environment, the negative sides of the water molecules.Then they cause a repulsive feeling between the central positive ion and the δ+)charges of the H atoms of the water molecules. There is a tendency to repulse H+.
A solution of, for example Iron(III)chloride can obtain in this way a rather acid pH value.
The drawing shows the attraction between Al3+ and the δ- part of Oxygen and the δ+ part of Hydrogen.
The water molecules (6 in total) surround the 3+ ion of Aluminium, because this positive ion attracts the δ- of water.
thus the distance between 3+ and δ+ becomes shorter, and with that, the repulsion between 3+ and δ+ becomes stronger.
The concequence is that the H can be (more of less) repelled. There could be even donation of H+ ionen, and all this we call: an acid character. (much more about this in module 9)
Normally the negative ions like to attract H+ ions (so the normally are bases).
Yet there are certain negative ions that can serve as acid and tend to donate H+. Example: HCO3-.