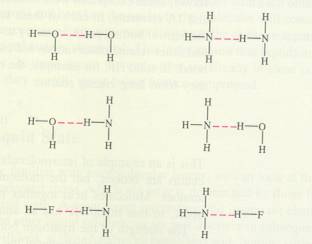
Hydrogen bridge
A Hydrogen bridge is another way for molecules to attract each other and to stay toghether.
This way of attraction has directly to do with the existance of dipole molecules, but it is just a bit more than that:
Apart from the presence of dipole forces, there are H-bridges:
A H-atomδ+ is situated in between two δ- -atoms.
Such a H bridge looks like an ionic bond, but in reality it has kind of covalent character.
δ- δ+ δ-
O - H - - - O
A H bridge can exist under the next four conditions:
- The whole complex must have a linear structure (all three atoms in one line).
- the presence of a Hδ+ also is a condition.
- The δ- -atoms must be present, one of them mostly being Oxygen (but not necessarily, it could also be Nitrogen)
- Those dashes (- - - ) indicat the bridge:
one pair of electrons of Oxygen came under the influence of Hydrogen; that's how kind of covalent bond start to exist.