Fire
In the ancient times the Greek (phylosopher Aristotle) developed a theory that all matter should be built up of (four) elements;
being:
earth, water, air and fire.
Att.: Those days they had a different concept of 'element' than we have in our modern society.
But nevertheless, they found that matter is constitued of four elements.
Well burning matter, like wood, must contain lots of the element fire; isn't that what comes out when burning wood?!
Solids must contain lots of the element 'earth'.
Liquids contain the water element.
If any matter start to bubble, that matter contained the element 'air'. etcetera.
It was the scientific way of thinking in those days.
Nowadays we know that fire is a form of heat energy that appears during exothermic chemical process; while that heat appears at one concentrated spot. Fire is a kind of high concentrated heat energy.
It is not really matter, although you may need particles to see the fire. In most cases, small particles are present in the hot spot; they start to glow and that causes the yellow color, sometime blueish. Also other colors can be observed.
The atoms of some substances involved in this high energy (substances that can be put into that fire) can - if not burned themselves - become 'excitated': electrons of those atoms receive temporarily some extra energy, and immediately donate again this extra energy. That outcoming energy often is radiation in the form of visual light, like blue, green, violet, yellow, etc. dependent on the character of the present atoms. (see module 1)
In the nineteenths century, European science knew a special theory about fire. They introduces a new concept: "phlogiston".
Phlogiston should be a volatile substance that escapes from a burning substance (a bit Greek thinking)
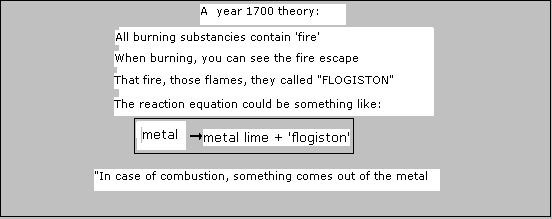
Below a chemical reaction that really takes place according to modern chemistry.
It turns out that nothing comes out op a burning substance. on the contrary!
Later more about this.
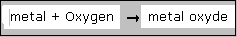
This phlogiston theory was rejected, because nor with deduction nor with practical research the existance of phlogiston could be proven.
Also they were able to measure that at burning an element, the product became heavier in stead of lighter.
So what: something comes out?
In experiments they determinated the mass of substances before and after burning.
Observations did absolutely not confirm the existance of something like phlogiston.
