Structural formulas
Every molecule has its own threedimensional shape. And only a good artist can really draw the good models.
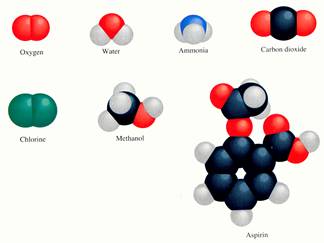
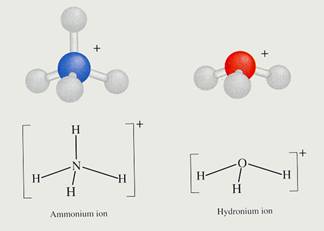
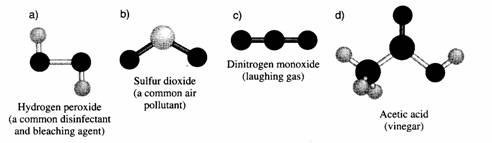
Not all chemists, teachers, student or pupils are great artists and they do not always have time to draw such beautyfull models as in the examples.
Well, you do not need to; a structural formula can help to show the composition of a particle in a very schematical way.
below a couple of rules:
- Every atom has and sticks to its own symbol;
- Every ion sticks to its own charge;
- Every covalent bond is seen as a dash (one for a single bond en two or three for a double of triple bond);
- If the atom might chose, than it prefers a connection to an atom of a different element;
(big exception is Carbon that loves to connect to its own kind)
- An atom of Hydrogen, directly connected to a carbon chain, often is left out; or you only show the dash in the structural formula.
In module 3 was also a part about how the electronic formula looks like the structural formula. the difference is the presence of absence of the valency electrons.


