no dipole
| methane | ammonia(g) | water |
| CH4 | NH3 | H2O |
| tetraedric no dipole |
dipole | dipole |
| b.p. 112 K | b.p. 240 K | b.p. 373 K |
| symmetrical | non symmetrical |
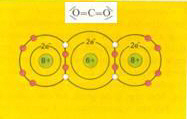 electronic structure of carbon dioxyde. There are real polar bonds, but in the total molecule the charges do annule each other. There is no dipole molecule. |
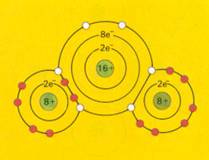 electronic structure of sulphur dioxyde. This molecule is non symmetric and the two polar bonds between S and O result in a dipole molecule. |