Indirect and spontaneous
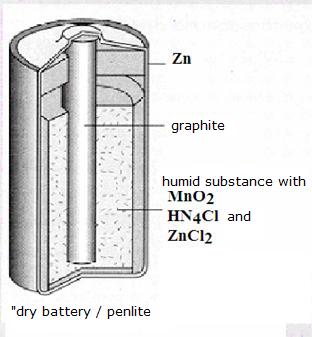
as seen in penlite's and batteries; electrochemical cells, galvanic elements
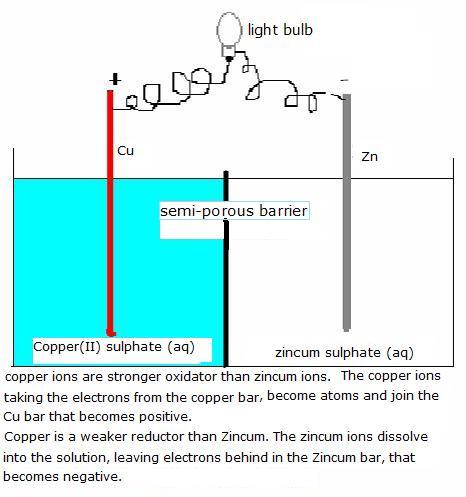
Spontaneous redox reactions normally occur in direct contact between the particles of the oxidator and the reductor, but can be realised in an indirect way, at a distance, at the surfaces of electrodes.
For example in the case of the Cu/Zn-electrode.
The zincum dissolves spontaneously, forming ions Zn2+, leaving behind the electrons in the Zincum bar that, because of that, collects a negative charge.
The copper ions in the solution capture electrons from the copper bar, become Cu atoms that immediatly join with the copper bar. This copper bar wil thus be charges positively.
The Zincum electrode becomes slowly thinner and de Copper electrode will grow a bit.
In the electrochemical cells (galvanic elements) occurs a spontaneous change of chemical energy onto electrical energy.
Strong substances react (oxidator and reductor) through electrodes (i.e. indirectly), while the weaker products are formed.
The electrons are transported and tranmitted via external conducting connections (wires and electrodes).
The movement of the electrones through wires is called: electric current.
We all know the applications as penlites and batteries.
Consider the following equilibria at two electrodes:
(at the anode:) red 1 (Cu)
 ox 1 (Cu2+) + electrons (a)
ox 1 (Cu2+) + electrons (a)
(at the cathode) ox 2 (Zn2+) + electrons
 red 2 (Zn) (b)
red 2 (Zn) (b)
If a dry battery delivers current (sends electrons), those electrons come from negative electrodes and move through the exterior to the positive electrode.
At equilibrium (a) the formation of Copper dominates (the equilibrium stays left).
As soon as the electrons arrive at this pole, the equilibrium can move even more tot the left.
At equilibrium (b) dominates the formation of Zincum ions.
As soon as electrons go away, this equilibrium tries to dislocate the equilibrium further, also to the left.
These two processes continue for a good time, as long as one of the reactants (Copper bar or Zincum bar) is gone.
We say than: the battery is 'empty'.
De batterij noemen we dan 'leeg'.
a penlite must have a strong oxidator and a strong reductor that do not have direct contact inside the battery. (they may not react directly)
The transfer of electrons must happen through the exterior, indirectly, so via the outside wiring; otherwise it is useless.