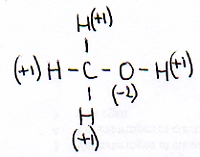Redox reactions in Carbon Chemistry
Also in the carbon chemistry there are redox reactions. Many carbon compounds are capable to donate or capture electrons.
att.: talking here about oxydation, we do not actually talk about combustion with Oxygen.
Most compounds in carbon chemistry can suffer combustion and form carbon dioxyde and water, but this is not now the topic.
As an example we take:
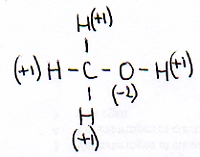
Normally an H in a compound has the oxydation number of +1 and the O has a Nox=-2
These two data you know, and also that the whole molecule is neutral.
This way you can calculate that the oxydation number of the C in this case must be: -2.
Polariteit
Oxydising a non polar carbon chain is very difficult (not talking about combustion, with Oxygen).
The division of valency electrons is in those chains very equilibrated and equal; there is no difference in electronegativity.
The presence of charges δ+ and δ- can cause nucleophylic and electrophylic attacks with redoxreactions as a consequence.
As soon as there is an Oxygen atom connected to the chain, there is polarity that makes redox reactions (transfer of electrons) more easy to occur.
The Oxygen atom, or any other atom with a good electronegativity, causes the needed polarity in that substance.
But note: not only polarity is needed; there must also be a position where more Oxygen atoms (or other electronegative atoms) can be connected.
A general rule is:
A C-atom in a carbon chain can be oxydises when that C simultaneously:
has an O ánd an H where an extra O can be connected between the C and the H.
In other words: To oxydise a carbonchain, there must be already an O-atom at the C-atom to oxydise, and also, that same C-atom must have at least one H-atom.
Normally, during the oxydation, an O is connected between the C and the H:
H becomes then: OH / In fact an H is substituted by an OH.
Mind:
Ether molecules have an O-atom between two C-atoms. There is a certain symmetria, so less polarity; in this case oxydation becomes more difficult.
A bonding between two carbon atoms is - in normal oxydation reactions - difficult to break; carbon chains normally remain unchanged (execpt in the case of direct combustion with Oxygen, where carbondioxyde and water are made).
Oxydizing various functional groups
Alkanes


Alkanes are difficult to oxydise, exept of course the burning with Oxygen.
Mind for example: natural gas, butagas, etcetera.
Alkanoles
Alkanoles are mostly oxydised with acid permanganate of with acid dichromate; i.e. with a rather strong oxydator.
Oxydizing an alkanol, the first step gives a substance with two OH-groups at one C-atom (check that).
As said, such a compound with more OH-groups at one C-atom is not stable; of those two OH-groups, one molecule of water will be removed.
Than remains a double bonded O (alkanal, alkanone, alkanoic acid).
We also can burn directly alkanoles/alcohols, and of course we obtain the well known carbon dioxyde and water.
But now we are talking about the more subtile way with weaker oxydators and with special products:
the ion dichromate in acid environment can oxydise a primary alkanol in two steps (via the intermediate alkanal) up to the final product alkanoic acid.
Alkanales


For the oxydation of alkanales (rather easy) we often use acid permanganate or acid dichromate.
Yet these oxydators are stronger than needed in these cases.
An alkanal has an aldehyde group (-CHO) and the C=O bond is rather polar. that's why oxydators easily attack the δ+-part.
Weak oxydators, like silver- or copper(I) ions are stong enough to oxydise alkanales, whereby the product will be a carboxylic group, that means an acid.
So: it is easier to oxydise an alkanal than an alkanol.
Alkanones

In the case of alkanones, there is polarity between the C and the O of the C=O group, but that particular C-atom does not possess an extra H-atom where the oxydation shoult have to take place.
So it doesn't work. Only with very powerfull oxydators can be done something, and alone if the C - C bond is broken.
Alkanoic acids
Organic acids normally cannot be oxydised easily, just like ethers and esthers.
Ethers
Yes, there is an O between two C-atoms, so there must be some polarity, but there is also great symmetry.
That's why we cannot expect big polarity, and therefore it will be more difficult to oxydise ethers.
It is difficult to oxydise organic acids. Not because there is no polarity, but because there is no H-atom connected to the polar Cδ+, where the O should be connected. The same for esthers.
Methanoic acid and dicarbonic acid can easily be oxydised.