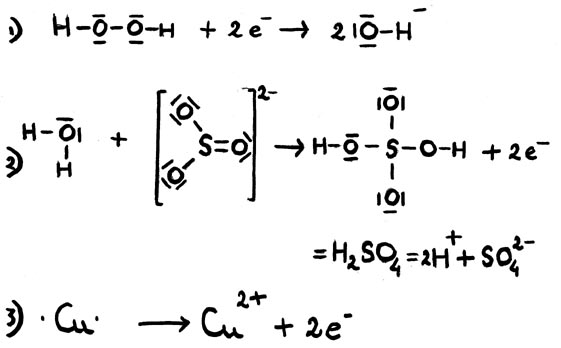Definitions
An oxydator is any particle that can/'want' accept or capture electrons;
it is an electron acceptor
[n.b.: 'want' is in brackets, because 'to want something' is not exactly a property of matter. Particles have nothing to 'want'.
Only living creatures can 'want'.
But nevertheless we use this kind of words in this course, just because it is fun to pretend that substances have that kind of properties.
Besides, we do not know everything about matter!!
A reductor is a particle that can or want donate electrons;
it is an electron donor
Electrons are not just stand alone available. They belong always to one or more particles.
Transfer of electrons, normally does not occur at distance; it occurs in direct contact between particles.
So a reductor cannot shoot an electron and somewhere else it hits an oxydator.
Some rules must be known for substances/particles that participate in a redox reaction:
- There are oxydators and reductors that only react if some auxiliary substances are present.
- Neutral elements very often are oxydators (in particular some non metals) or reductors (many metals).
this has everything to do with the fact that metal atoms have few valency electrons, and non metals have a lot.
To study this, you can go to module 03 about the chemical bondings and to module 01 about the atoms.
Lots of redox reactions occur, also in daily life, such as burning of fuels (petrol with Oxygen, or rusting of Iron), but also such as the energy production in the human body. Its all kind of combustion.
Nevertheless, most redox reactions of this course are not as such the combustion reactions.
In daily life, exept from Oxygen, there are many other oxydators (and reductors): Iron, glucose, permanganate, Hydrogen, Alcohol, etcetera.

More definitions:
A redox reaction is a chemical process
where electrons are transferred from one substance to another.
or else:
A redox reaction is a chemical process
in which the oxydation numbers of one or more elements change.
Any substance that accepts electrons during a reaction is an oxydator (Ox)
The opposite: a substance that loses electrons is a reductor (Red)
During a redox reaction always react a reductor and an oxydator.
electrons are transferred from the reductor to the oxydator.
In this transfer, always the valency electrons are involved (the electrons of the outer orbital of the atoms)
Some examples:
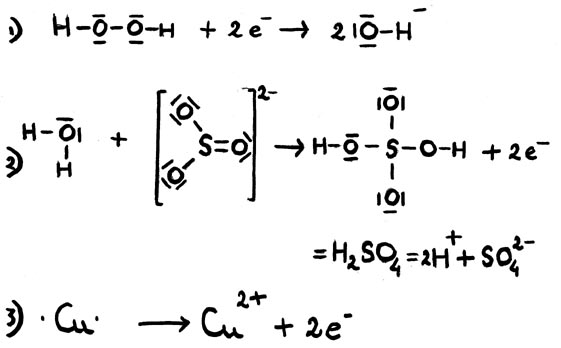
In the middle example, a sulphur atom donates two electrons and act as a reductor, where sulfate is formed from sulfite.