Reactant, reagent, product, side product, overdose
Substances in a chemical reaction react or are made, always in a fixed proportion.
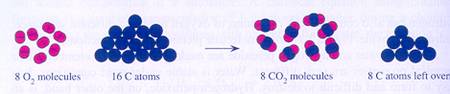
A reaction between reactants continues as long as there are substances available. It stops when one of the reactants is gone.
The rest just remains, and can no longer react; it was present in 'overdose'.
You must understand that it is wise to calculate, on beforehand, how much you need of every substance in a reaction.
Only with good calculations you can avoid that any substance remains without options. On top of that, the remaining substance could easily complicate the reaction.
Calculating well is a first condition in a chemical reaction.
Another disadvantage of badly / no calculating (reaction calculations) is that the products at the end can be polluted.
Many reaction occur in the presence of substances that do not really participate in the proper reaction.
Side products
In the lab or in the chemical industry, we like to produce chemical products.
A general problem is, that, besides the aimed main products, many times always are formed so called side products,
mostly unavoidable because the reaction does produce also those substances.
Maybe this will not be a problem, but often purification is needed, and that can be rather complicated. Its a nasty problem.
Question 8
Precipitation reation:
NaCl(aq) + AgNO3(aq)
 AgCl(s) + NaNO3(aq)
AgCl(s) + NaNO3(aq)
In this reaction, the reactants Ag+ + Cl- change into the product AgCl(s).
Apart from the meant final product, side products are made: NaNO3(aq).
This is a simple example of a side product.
