Polycondensation
Many molecules connect to each other, where lots of water molecules are formed as a side product (or sometimes another small molecule)
A macro molecule is produced via a condensation mechanism.
example:
Glycol C2H4(OH)2,
with two OH-groups per molecule, can suffer a polycondensation process in two directions.
HO – CH2 – CH2 – OH
Each OH-group can react with the OH-group of another molecule. Every time water is formed.
The glycol molecule can extent to two sides.
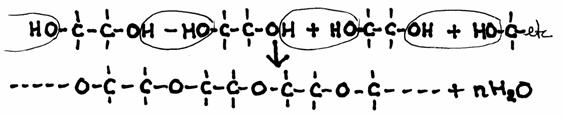
The product is, in this case, a polyether, a solid, while the reactant glycol is a liquid.
Famous polycondensation products are:
- Polyethers
- Polyesters
- Polypeptídes
- Polysaccharides
Copolymerisation is the process where participate more than one kind of monomers.
In the industry many copolymeres are invented, made and applied.
For example, the material of videotapes are a copolymere product of the two monomeres:
glycol (1,2-dihidroxyethane) and tereftalic acid (1.4-dicarboxylbenzene ).
Question 24
Copolymerisation of the monomeres glycol and oxalic acid is possible, with a little bit of concentrated sulfuric acid as a catalyst.
This reaction starts with an attack of protons at oxalic acid, whereby a carbonium ion is formed (a C with a positive charge).
This is the slow step of the reaction. The slowest step determines the total reaction rate.