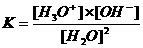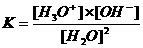Kw
In module 9 was spoken of water as an ampholyte, so a very weak base ánd a very weak acid.
If no other substances are dissolved in water, than the pH of that water, at 25 degrees C is exactly 7 as also the pOH = 7.
With the (water)equilibrium (H2O + H2O  H3O+ + OH-) comes of course also an equilibrium condition K:
H3O+ + OH-) comes of course also an equilibrium condition K:
The waterconcentration is constant, does not change.
The water concentration is about 55.5 mol/l and the dissociation practically does not change anything.
From this you can draw a relation:
K.[H2O]2 = [H3O+].[OH-]
 K·55,5 = Kw = 10-7·10-7 = 10-14
K·55,5 = Kw = 10-7·10-7 = 10-14
Kw is called the water constant and is, at 25°C : 10-14
As in all equilibria, also the water constant depends only on the temperature.
The above formulas can also be written with p-values:
pKw = pH + pOH
or in numbers:
pKw = 14 = 7 + 7
If the pH and the pOH equal each other, so the [H3O+] and the [OH-] are equal,
than the solution is neutral.
Because Kw is a constant (so pKw too) pH and pOH toghether must be 14.
example: pH = 5 and pOH = 9. [att: only at room temperature!]
If the pH becomes bigger, the pOH becomes smaller and v.v.
- adding acid makes the [H3O+] larger, so the pH <.
- adding base makes the [OH-] larger, zo the pOH <.