Measuring pH

Study carefully the picture.
The pH-meter (blue) shows a valueof 2.7: here we've got a vinegar solution (the yellow liquid in the beaker).
Next to the solution is the bottle with the rest of the vinegar (almost empty).
The small plastic tube close to the pH-meter is a protection cover of the electrode.
The electrode itself is immerged into the vinegar (normally the electrode is kept in that cover).

This photo can be converted into a table with three columns and three rows.
|
phenolphtaleene |
methyl red |
| ammonia(aq) |
carmin red |
yellow |
| HCl(aq) |
colorless |
red |
Indicators are weak acids with weak conjugated bases (present in equilibrium).
The structure of an indicator mostly is an organic and rather complex molecule.
Very often we use the short formula: HIn.
That H is of course the proton that can be donated (the indicator being a weak acid) and the rest - In - is the biggest and complex part of the molecule.
In module 8 we already used an example of a substance that has the opportunity to visualise the location of the equilibrium.
That was indeed an indicator.
The acid base indicator is applicable as such because HIn has another color than In-.


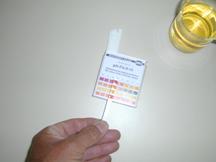
We measure the pH-value of vinegar with universal indicator paper. One paper strip is hold for a moment in the vinegar.
Four indicators on the strip get their own color each, and the combination of those four must be compared with the standard colors on the box.
In this case the four colors correspond with a pH of about 3 (not easy to see on the picture)
Besides the universal indicator paper their are various other types of indicator paper.
Sometimes the colorants are completely vegetable.




