acid-base-calculations
Calculations with pH
The most imporatnt reason to use p-values is that we often apply concentrations of very diluted solutions.
It is much easier to say:
pH = 6 than [H3O+] = 10-6 mol/l.
Please don't ever forget tjat a high p-value always automatically means a very small corresponding real value.
pOH = 9 (a rather high value) means a low concentration of OH--ions:
pOH = 9 → [OH-] = 10-9 mol/l
Saying of writing something about concentrations, the unit mol/l (mol per liter) may not fail; using the p-value, there is no need for any unit.
But mind you: the p-value is based upon the well known unit mol/l
To remember: in water with a normal temperature (say 20 - 25ºC): pH + pOH = pKW = 14
So, as soon as you know pH, you also know pOH.
An aqueous solution is called NEUTRAL when pH = pOH, at whatever temperature. That is the most important criterium for a neutral solution.
It is similar to say: the concentrations of H3O+ and OH- are equal.
Adding acid to a solution means that the pH-value becomes lower and the pOH-value increases.
Adding a base means a higher pH and a lower pOH.
The values of pH can also be broken number, like 3.4 and 10.7 e.d.
This can difficult the mathematical calculations.
For example: if the pH=3.5, then the concentration [H3O+] equals 10-3,5mol/l.
But often we do not accept broken exponents.
You have to see and understand immediately in this example that the concentration must be between the values 10-3 and 10-4mol/l (because the pH is between 3 en 4).
A calculator of course immediately gives you the solution, but even without the machine, you must be able to execute the calculation:
pH = 3.5 = 4 – 0.5 → -log[H3O+] = 4 - 0.5 → [H3O+] = 3 x 10-4 mol/l.
Calculations with KA and KBand with pKA with pKB
Exercise:
Calculate the pH of the following solutions:
- 0,1M HAc
- 0,1M NH3
- 0,1M HCl
Answer a):
0.1M HAc means: 0.1 mol acetic acid (CH3COOH) was dissolved in water with an final volume of 1 liter.
A part of the acid molecules dissociate in H+ and Ac-.
The amount of H+ (in water H3O+) determines the value of the pH (=-log[H3O+]).
We must know this amount of H+, just like the strength/weakness of the acid, or: the KA
We consult the table to see that KA = 10-4 or pKA = 4
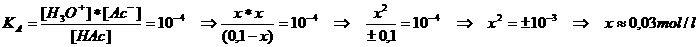
We know that HAc is a weak acid. The value of x will be small compared to the [HAc]
or: x can be neglected.
So: 0.03 mol HAc dissociated into ions → [H3O+] = 0.03 = 3 x 10-2 mol/l → pH = 2-log3 = 1.5
Never forget:
The weaker an acid, the smaller KA
The stronger an acid, the bigger KA
you can check this statement in table I.
Att.:
Strong acids and bases in the table do not have a K-value.