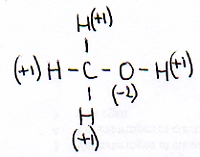Oxydation number Nox
In module 10 the redox reactions were treated, including the oxydation number. You know that during a redox reaction the oxydation numbers of some elements will change.
In redox reactions in the carbon chemistry, such an oxydation number not always can be easily defined.
Where exactly occurs the transfer of electrons, that is the question, in particular regarding the carbon atoms.
The redox tables do not provide halfreactions of the carbon chemistry, so the changes of the oxydation number in these cases must be discovered by yourself.
As an example we take:
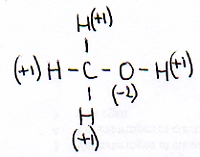
Normally an H in a compound has the oxydation number of +1 and the O has a Nox=-2
These two data you know, and also that the whole molecule is neutral.
This way you can calculate that the oxydation number of the C in this case must be: -2.