Organic acids
There are many of them, and the most important for this course are those with a carboxyl group (the alkanoic acids).
Phenol is another kind of organic acid.
Now we talk about carboxylic acids, amino acids, fatty acids and phenol, but also about bases.
Much more about this item can be found in module 11, organic reactions.
Here only some questions:
Consider the structure of a carboxylic group.
The carboxylic group is not a strong acid. Methanoic acid might be one of the strongest.
Comparing methanoic acid with trichloro methanoic acid, then you must know that the last one is stronger.
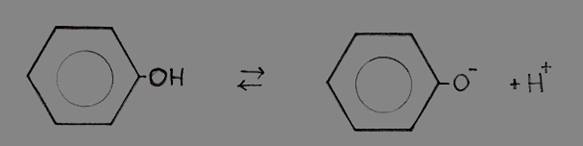
Phenol (benzene with a hydroxyl group -OH) is a liquid with a weak acid character.
Hydroxyl groups are mostly found at alcohols and are normally not acid at all. Byut the one of phenol has the option to donate the H+
of that hydroxyl group.
This remarkable fact is due to the presence of that benzene ring (with six free electrons in the ring).
The absence of the OH-proton, meaning the presence of the O- at the ring, increases the freedom of those six (+1) electrons; that makes the whole complex even more stable.
Losing the H+ strenghtens the stability of the structure; from there comes the (weak) acid character of phenol.
or
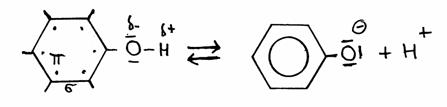
Phenol  phenolate + H+
phenolate + H+
The exra elecron of phenolate will participate in the free movement of the six electrons of the ring, thus stabelising the phenolate.

