Solubility
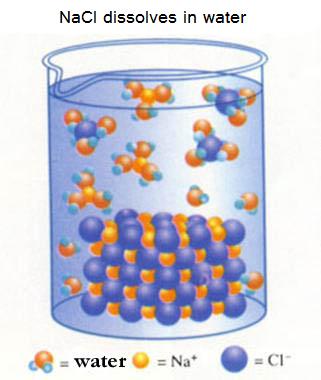
ionic substance that dissolves in water: Sodium chloride
| Na+
|
Cl-
|
Na+
|
Cl-
|
Na+
|
| Cl-
|
Na+
|
Cl-
|
Na+
|
Cl-
|
| Na+
|
Cl-
|
Na+
|
Cl-
|
Na+
|
| Cl-
|
Na+
|
Cl-
|
Na+
|
Cl-
|
| Na+
|
Cl-
|
Na+
|
Cl-
|
Na+
|
| Cl-
|
Na+
|
Cl-
|
Na+
|
Cl-
|
The ions Na+ and Cl- escaping from a lattice (this happens at the surface of the cristal) immediately are surrounded by water molecules (because of polar attraction forces).
The ions still attract each other, but not enough to come back in a lattice. Those water molecules make that impossible.
In this case, we have a soluble salt.
Other ions, smaller ones or more charged, also surrounded by water molecules (=hydrated), often can come toghether again and form a lattice. Their mutual attraction is big enough to succeed in that.
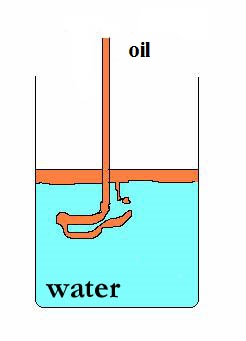
heterogeneous mixture: (example) oil + water
The polar molecules of water attract each other, while the non polar oil molecules stay and stick toghether. Mixing water and oil molecules has no success.
General rule:
- polar substances attract polar substances (like sugar that dissolves well in water)
- non polar substances attract non polar substances (like fat in petrol)
- polar substances do not mix with non polar substances (like water and oil)
ANY KIND LOOKS FOR ITS OWN KIND

