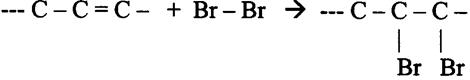(Un)saturated
In nature the lipids (fats)are the mono, di, and triglycerides of fatty acids, or also: the esters of glycerol and fatty acids.
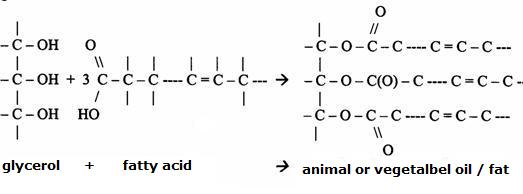
"Fat" normally is saturated.
Do we use unsaturated fatty acids: than the fatte character decreases and the oily character increases.
Oily substances, normally, are more unsaturated than fats.
Unsaturated substances have one or more double bondings in the chain, and can be saturated, for example with Bromine (Br2)
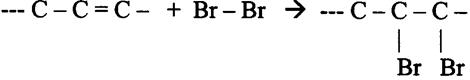
A layer with unsaturated oil (yellow color)  layer with fat (diffuse)
layer with fat (diffuse)
A layer with Br2(aq) (yellow color)  a layer of water without Bromine (colorless)
a layer of water without Bromine (colorless)
"Normally, margarine is made of vegetable oil. That oil is build up of unsaturated molecules.
The problem is dath oil is a liquid and how to spread this on your slice of bread?
We can resolve that problem (we did that in the old days) by saturating the double bonds with Hydrogen (catalyst = Zincum).
More saturated means: harder, so it got a harder constitution.
But... in the same process, this substance is losing its healthy character (unsaturated).
The new way to change a liquid into a solid is the application of emulgators.