Monosaccharides
The monosaccharides can occur in a linear or in a cyclic configuration (above you only see cyclic forms).
Below you can see how glucose can have the linear and the cyclic form:
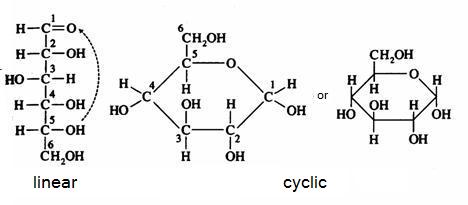
The linear structure can easily be oxydised, with a weak oxydator; often we use for that:
Ag+ ammonia-silver-solution = reagets of Tollens. Another one, Fehlings' reagent, also acts very fine (Cu2+)
The cyclic configuration is not easily oxydised (there is no =O bond available).
In practice the two structures, the linear and the cyclic, in a solution, are in an equilibrium with each other, so the linear structure is than always present and always ready to suffer oxydation.
Than the linear structure disappears in that process, the equilibrium will shift from the cyclic form tot the linear form (that continues to suffer oxydation), and at the end, all the monosaccharide is oxydised.
glucose and fructose appear in two ways, the linear and the cyclic. The two configurations are - in aqueous environment - always in equilibrium with each other.
And as said, only the linear structure has the right group to oxydise (the reductor group).
During the redox process not only the linear form (the reacting one), but also the cyclic form will disappear.
