Michaelis Menten
(there is another paragraph about the same topic, an extra explanation from literature in 5.7)
Every reaction occurs with a certain reaction rate V and with its own reaction constant k
k1 k3
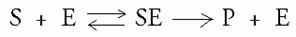
k2
Starting with three presupposition supposing ideal / optimal conditions, you may state that the rate, at its maximum, is Vmax

The three presuppositions are:
- a steady stat wherein the concentration of the intermediate ES does not change; the production of the ES complex takes place with the same rate as its disappearance;
- Every present enzyme participates;
- The system is in an optimal environment (saturated with S, the best pH, the best temperature);
In this way the reaction rate V has ist maximal value Vmax
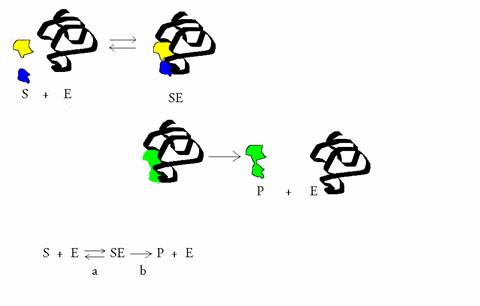
about the rate of producint the intermediate ES:

about teh rate of the disappearance (to the right and to the left) of that intermediate ES:


KM is the constant of Michaelis
KM shows the affinity of the enzyme for the substrate; a low KM means a lot of ES, or, a lot of substrate S,
and that means: a low KM and a bigger rate.
The relation between the reaction rate and the KM can be seen in the Michaelis Menten equation:

The values of KM can vary between 0 and thousands.
For a certain standard amount of hexokinase or glycokinase, we measure the production rate (V) of glucose phosphate (The product G6P) as a function of the glucose concentration [S].
The results show that Vmax of the two enzyms are equal (±100 nmol G6P are produced per minute), but that the Km-values differ considerably:
Km of hexokinase = 0.1 mM, Km of glucokinase = 10 nM (factor 100)."
Have again a good look at the following equilibrium with the intermediate SE:
k
1
k
3
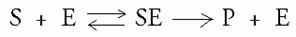
k
2
The constant of Michaelis
 shows the affinity of the enzyme to the substrate.
shows the affinity of the enzyme to the substrate.
The equation of Michaelis-Menten
 shows the relation between KM and the reaction rate.
shows the relation between KM and the reaction rate.
We know various types of enzyme inhibitors:
- product inhibitors
- drugs inhibitors
- allosteric inhibitors



