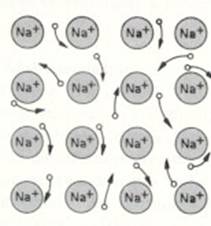
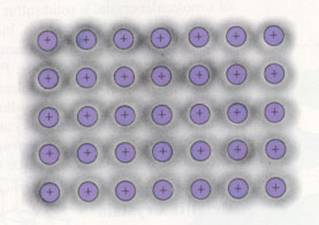
Metallic bond = attraction between (negative free) electrons and (positive) metal ions in a lattice
|
These attraction forces can be considerably strong. But can differ per metal.
To break a lattice of Iron (i.e. to melt Iron) you need very high temperatures, but the metal Lead melt much easier. (see module 5)