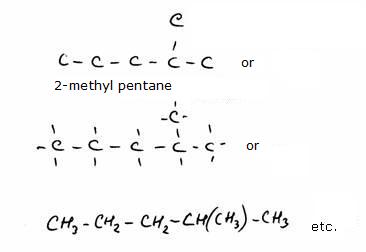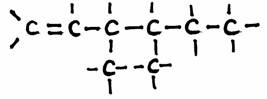Carbon chains
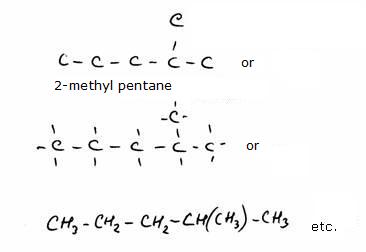
The amount of substances in the organic chemistry (carbon chemistry) is unimaginable large.
The most important reason for that is the opportunity of Carbon atoms to connect to each other and thus to form short or very long carbon chains.
A good nomenclature (namegiving)with basic rules for all those chains is absolutely necessary.
those chains can be met in chemistry books in different ways. Sometimes they are drawn very detailed, with every atom visible, sometimes
you meet them as a kind of skeleton where not all atoms and/or bonds are shown.
Without knowledge of the basic rules it is not possible to understand the famous 'international nomenclature'.
Main chains and branches
As a consequence of its special charactyer, the C atom can form chains with simple, double, triple or even cyclic bondings, not only with Carbon, but with many other atoms.
There are so many different molecules based on the Carbon atom that a carbon chemistry has been introduced.
In the old days (but you still meet that word often) we called that organic chemistry.
primary, secundary, tertiary en quaternary
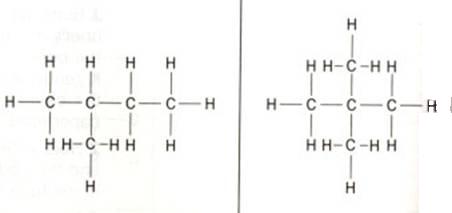
The structure below shows 'methyl butane' at the left and 'dimethyl propane' at the right side.
Cabon atoms that are only connected to one other C-atom are called: primary C-atoms/ they always are situated at the end of a carbon chain.
C-atoms in between two others, we call secondary C-atoms.
Thus we also have tertiary C-atoms, connected to three others, and even quaternary ones, surrounded by four other C-atoms.
The words primary etc. are not exclusively used for the C-atoms. Also other atoms or atom groups can have this qualification.
For example, if one OH-group is connected to a secondary C-atom, we speak of a secondary alcohol, or a secondary hydroxy group.
In proteins you also will find those concepts primary etc. but then they mean something different.
Nomenclature
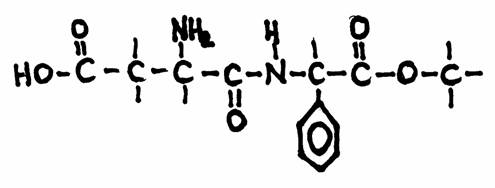
This is the structuere of 'aspartame', a substitute for common sugar for persons that must take care
(diabetici, of people afraid of becoming fat).
Aspartame has an effect 100 times stronger than common sugar.
The structure has many functional groups.
The organic substances always contain a main chain of Carbon atoms.
In the nomenclature the fist thing to do is: search and recognize the main chain.
- Always chose as a main chain:
- the longuest possible chain, but at the same time:
- the chain containing possible double or triple bonds.
- The main chain can have brances or functional groups, like an OH-group
- If necessary, we add numbers to indicate to what position in the main chain the brance, the special bond of the functional group is connected.
Always try to achieve the lowest possible numbering.
Rules for the nomenclature, here for aliphatic hydro(gen)carbons
Every carbon compound has a main chain based upon the number of C atoms in the main chain:
Met..., et..., prop..., but..., pent..., hex..., hept..., oct..., non..., dec..., in general: alk...
on the dots appear suffixes:
- ane: the main chain only has simple bonds.
- ene: the main chain also has double bonds.
- yne: the main chain also has triple bonds.
- yl: this is used when talking about a branch, not a main chain.
Examples:
Propene is a molecule with a chain of three C atoms with one double bond.
The positions of double and triple bonds, if necessary, are indicated with numbers.
3-etyl is a branch, a side chain of two Ca atoms, and is connected to the third C of the main chain.
Apart from the suffixes, there are many prefixes:
- Mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca
they indicate 1 to 10 and give the number of a certain group.
- Cyclo
here the main chain is not linear, but circular.
Example: 3,4-dimetyl,1-hexene:
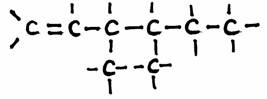
N.B
H atoms can be left out in the structural formulas.
De homologous series
Such a series contains substances with one kind of functional group, with the same general formula and with similar properties.
Example: all alcohols have an OH-group, and a general formula CxHyOH.
But: you have also ethers and esters etc.
The differencies between the membersof a homologous series depend on the length of the carbon chain CxHy and other data.
Methanol, for example, will evaporate much easier than propanol. But chemically they show very simmilar reactions.
(un)saturated
A carbon chain can be saturated or unsaturated, depending on the presence of double or triple bonds between C atoms.
Later we return to the typical reactions of unsaturated bonds (that can be 'opened' to become saturated; something is added).