column chromatography
In column chromatography we fill a glass tube (can have various sizes) with the fixed phase.
On top of this column we introduce the mobile phase ((l) or (g)), and at the bottom (small tap) that mobile phase can escape.
A mixture of components is taken with the mobile phase from top to bottom, and the rate of passing the tube varies with each component.
An advantage of this methode is that the we can regulate very well the passing rate of the mobile phase, and also that with a detector at the bottom of the tube we can detect the passing of each component (and even how much of it).
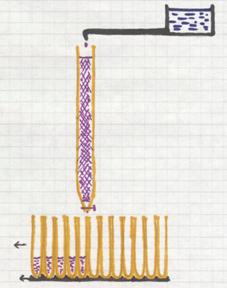
A special type of chromatography is the colomn chromatography. The column (a glas tube) is filled with a special solid (sand, aluminium oxyde, certain gels, e.o.). On top we place a mixture of substances, that enters into the solid filling of the column. Immediatly after disappearing of the mixture, you open a tap that drop by drop put a solute on the column. During the passage of this solute down through the column, the mixture is taken with it. But which component of the mixture will pass fast and which one will pass slowly, that depends on a number of factors, such as: how big are the particles of the component? how soluble is the component in the solute? How well is the component absorbed at the column solid? etc.
From time to time (seconds or minutes) a new test tube can be put under the column to catch the outcoming solute. At the end of this proces, every test tube contains a bit of the solute + in some tubes a bit of a component. There are fine detection methods to find those components.
Question
One of the separation mechanisms for particles, in columns, is based on the difference in largeness of the particles.
Which particles will leave the column first: the big ones or the small ones?
