Cation acids
The most famous example is the Aluminium ion dissolved in water, but there are lots more.
Cations normally are hydrated polypositive metal ions:
Al(H2O)63+, Cu2+, Fe2+ of Fe3+,
any many more, and always hydrated in water.
hydratation
The poly positive ions attract closely, in watery environment, the negative sides of the water molecules.
Then they cause a repulsive feeling between the central positive ion and the δ+)charges of the
H atoms of the water molecules. There is a tendency to repulse H+.
A solution of, for example Iron(III)chloride can obtain in this way a rather acid pH value.
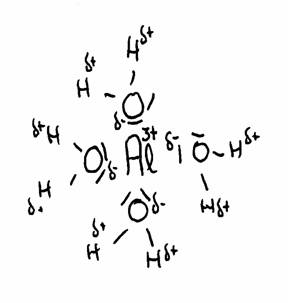
The drawing shows the attraction between Al3+ and the δ- part of Oxygen and the δ+ part of Hydrogen.
The water molecules (6 in total) surround the 3+ ion of Aluminium, because this positive ion attracts the δ- of water.
thus the distance between 3+ and δ+ becomes shorter, and with that, the repulsion between 3+ and δ+ becomes stronger.
The concequence is that the H can be (more of less) repelled.
There could be even donation of H+ ionen, and all this we call: an acid character. (much more about this in module 9)
