molar gas volume
To know the volume of a gas, in particular the volume of one mol of that gas, then the calculation is different.
There is a fundamental difference between gases on one side and solid / liquids on the other side:
The most special characeristic of gases is that all gas particles are lose from each other and that therefore the volume of a gas does not depend on the size of the gas molecules.
The issue is: how far are those molecules separated from each other? How big is the distance between the molecules? What's the distance? That's what counts.
in solids en in liquids, all molecules close toghether, wether moving or vibrating or quiet. So in (s) or (l) the first question is: how big are the molecules? The bigger, the more volume they need.
But with gases totally different.
The volume of a gas, in fact the mutual distances between the gas molecules, in the very first place is determined by pressure and temperature:
- At higher pressure the gas molecules are forces closer toghether and the mutual distances are smaller, and you have more molecules per liter, i.e. more mols per liter. That will make a gas with high pressure also heavier.
- At higher temperatures (i.e. the gas particles move faster and with higher rate) the molecules will have more distance. then you will have less molecules per liter, or less mol/.
A liter hot gas is also lighter then a liter cold gas.
At 0°C and 1 atm. pressure, the volume of 1 mol gas = 22.4 liter, no matter what gas. At another temperature and pressure that volume is different, but for any gas the same at the same conditions.
for example: at 100°C every gas has a volume of about 27 liters.
There is a general gas law. With that you can calculate how many liters make one mol gas, at a defined temp and pressure.
We've got for that mathematical formulas.
Taking an equal volume of two gases with the same temp and pressure, then the same number of moles is found as well as the same number of molecules, whatever gas you consider.
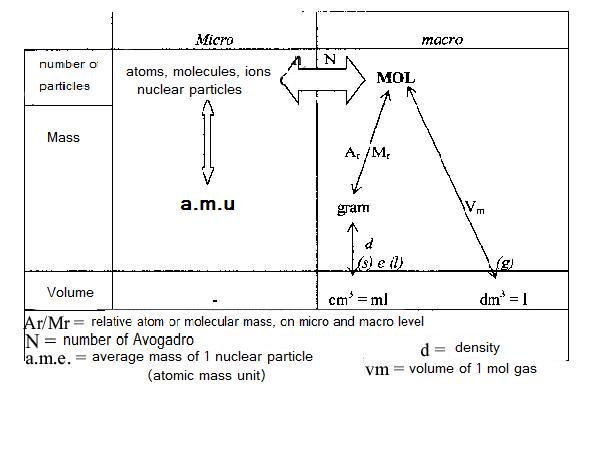
When changing units in chemical calculations, the concept of MOL always is in the centre. You must learn the scheme below, and know how to apply it: