Formulas
In the chemistry system, all substances have a name, but there are different types of formulas:
- Every element has its own symbol (that is also a chemical formula!). Here we have the basic formulas.
Note that they are always built up of one CAPITAL, often combined with one small letter.
examples: Cu, H, Co, Ar
- Many elements can make simple ions, and those ions have simple ionic formulas.
A couple of examples of that: Cu2+, Fe3+, I- and Cl-
Behalve de eenvoudige, zijn er ook complexe ionen zoals bijvoorbeeld NH4+, PO43-,H3O+, etcetera.
- the biggest part of the substances has a molecular formula;
they show the real number of atoms of everyt element in such a molecule.
examples: H2O, C2H5OH.
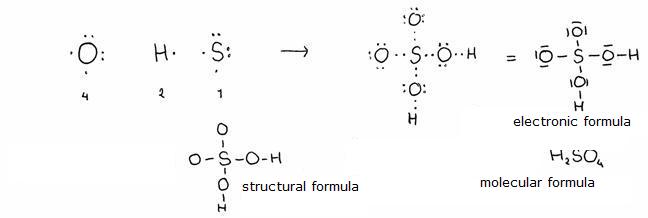
- The electronic formula shows an atom or an ion or a molecule toghether with all its valency electrons (with dots or slashes), including the shared and non shared electrons.
- then there is the structural formula, that in fact equals the electronic formula without the shared electrons (electron pares)
- A formula that simply shows the proportion of the constituting elements, we call empiric formula.
examples: NaCl, Cu, CaO, enz.
The empiric formula is applied in the case of substances with ions and with metals.
N.B. The number 1 is never written in formulas.
Example:
We write Na1Cl1 or Cu1S1O4) simply as NaCl and CuSO4
