Phenol
There is a special type OH-group that can function as a weak acid.
We don't talk here about ordinary aliphatic oH-groups, but about the aromatic OH-group connected to a benzene ring.
Such a benzen ring with an OH-group is called: phenol.
| C6H5OH |
+ |
OH- |
 |
C6H5O- |
+ |
H2O |
| acid |
|
base |
 |
conjugated base |
+ |
conjugated acid |
In structures you see that phenol donates in the following way:
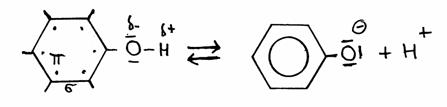
phenol phenolate
Special is that in phenolate all valency elctrons of Oxygen, toghether with the electrons op the type π in the ring, come to a kind of resonance state.
The negative ion becomes therefore more stable.
The consequence is that phenol stays with the tendency to donate a H+, much stronger than a normal aliphatic OH group.
The hydroxyl group and hydroxy benzene
You already know that an OH group only can lose / donate H+ (react as an acid) if that C-atom at the same time is connected with a O (so talking about a carboxylic group).
The aliphatic hydroxyde group, that is an alcolhol group, normally is not an acid nor a base.
The alcohol group is not basic nor acid.
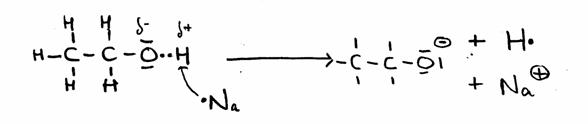
Only in the case of very agressive, reactive substance, like Sodium, the OH group can donate its H.
CH3 CH2 OH + Na·  CH3 CH2 O- + Na+ + H·
CH3 CH2 O- + Na+ + H·
As soon as 2 H·-radicals exist, a H2-molecule can be formed, so a gas.
The other product is called Sodium ethanolate, a stong basic substance.
In structures: