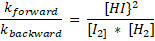The equilibrium constant
Vforward = kforward x [I2] x [H2]
and
Vbackward = kbackward x [HI]2
We know that at equilibrium, the forward and the backward reaction occur in the same rate:
Vforward = Vbackward
Or:
kforward x [I2] x [H2] = kbackward x [HI]2
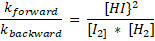
Att.: the quotient of the two constants gives one new constant; we may write:
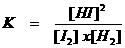
K is called: "the equilibrium constant"