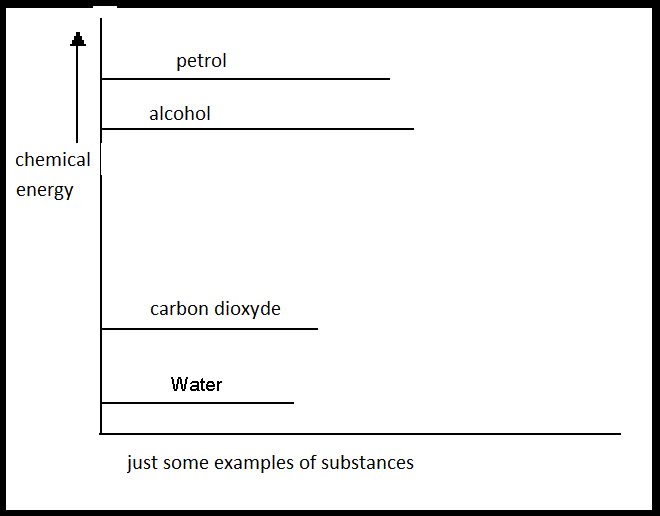
| I | The products have more energy than the reactants | In this case de products have gained energy; this is only possible when the system gained energy from outside (ΔH > 0). |
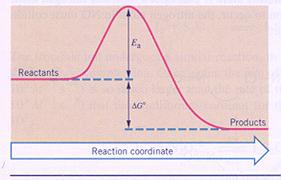
| II | The products have less energy than the reactants | Here the reactants lost energy; the system has donated energy to the outside environment. (ΔH < 0). |
| III | The products have the same energy as the reactants | In this case there is a chemical equilibrium. (ΔH = 0). |