Analysis of elements in Carbon compounds
A carbon compound (organic chemistry) always contains the element C, often H and sometimes O.
Here we leave other elements like N and S out. The formula of the substance to investigate could be, for example: CxHyOz
In the analysis of elements, you must determine the amounts of C and H in (milli)grammes.
Because you measured the total mass of the substance beforehand, you automatically can see if Oxygen was present, and, if so, how much.
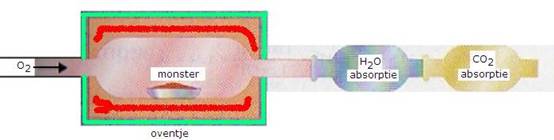
- You weight very carefully and exactly a certain amount of the substance to be analysed (a monstre).
Then this substance must be burned completely; all C is transferred into CO2 and ll H into H2O.
- The amount of Carbon dioxyde and water must be weighted.
- From the amount of carbon dioxyde can be calculated the amount of C
- From the amount of water can be calculated the amount of H
- If the original amount of substance is more than C + H toghether, then there must have been Oxygen in it.
Now you can check of Oxygen was present in the original substance; if so, you can write that amount.
Now you know the mass proportion C : H (: O)
That mass proportion must be transferred into the mol proportion, by dividing by the atom masses of C, H (and O).
In that way you get:
the proportion x : y : z
and the proportional formula (CxHyOz)n,
and not the real molecular formula CxHyOz.
The carbon dioxyde and the water that must be determinde in this analysis of elements, can be captured and weighted as follows:
- First the water(g) is captured by a hygroscopic substance that was weighted before the experiment.
For that you could apply: waterfree Calcium chloride or waterfree Copper(II)sulfate.
- Then the carbondioxyde is captured by, for example, a strong base like Calcium hydroxyde.
Again that must be weighted before and after the experiment. The difference is the captured gas.
