Electrode potential
You already know the next image of the voltaic cell.
Such an electrochemical cell aims to create electrical energy with strong chemical substances (oxydators and reductors).
We always need two redox couples (to be found in the redox tables)
The (half)potential of any redox couple can be measured, and its values you can find in most tables; they are based on standard circumstances: 25oC and 1 atm. gas pressure or a concentration of 1M.
The stronger an oxydator or reductor, the higher the delivered potential.
Joining two couples in a complete cell, there will be a difference in potentials, a voltage, between the two redox couples.
In the case of a penlite that voltage will be about 1.5V.
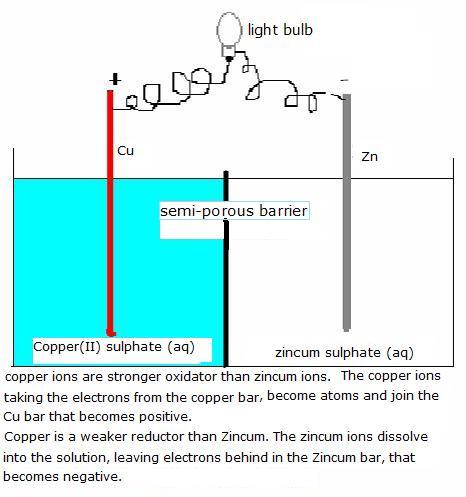
Here we have two halfreactions of two redox couples:
Red: Cu Cu2+ + 2e-V(standard) = +0,34 V
Cu2+ + 2e-V(standard) = +0,34 V
Ox: Zn2+ + 2e-  Zn
Zn
V (standard) = -0,76 V
The final voltage, under standard conditions, is: (red - ox) =0.34 - (-0.76) = 1.10 Volt [att.: you must always subtract the smallest from the biggest]
This battery wil create a voltage of 1.1 volt.
Changing the conditions will change the voltage. A higher concentration of Zn-ions (more OX) probably will create a higher voltage.
