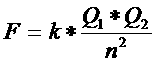dipole moment, polarity
How atoms may have a tendency to attract negative particles (mostly electrons), is called ELECTRONEGATIVITY (see module 3).
The Electronegativigy is abbreviated with the letter E.
Wether or not electrons (negative particles) are attracted easy or difficultly by neutral atoms (and possibly been 'eaten', depends much on the nuclear (postive) charge and the atomic ray (distance) of that atom.
The bigger the nuclear charge, the stronger the attraction forces on the negative particles.
The bigger the distance of the negative charges to the nucleus, the weaker the attraction forces.
An atom of Chlorine will attract electrons stronger that an atom of Iodine.
In the Periodic Table, the nuclear charge increases from left to right, while the number of main levels remains te same. The outmost level, the outer shell will be attracted stronger, and the atom becomes smaller from left to right.
The consequence of this is that the attractin force of the atom to the negative particles outside the atom will increase, so the E(lectronegativity) in one Period will increase from left to right.
The atomic ray increases in the Periodic Table from top to down, because every time there is an extra main shell.
Negartive particles (like electrons) will be attracted every time weaker from top to down, because every time the distance is bigger.
If a molecule has an non symmetrical distribution of charges, then that molecule does have a so called 'dipole moment":
dipole moment = charge x distance
With that distance we mean the distance between the centre points of the charges.
The dipole moments of the four Hydrogen Halogenes are as follows:
|
|
bonding distance
|
dipole moment
|
| HF
|
90 x10-12m
|
6,4
|
| HCl
|
130 x10-12m
|
3,5
|
| HBr
|
140 x10-12m
|
2,7
|
| HI
|
160 x10-12m
|
1,4
|
If the dipole moment of a molecule equals 0, then this substance is non polar; if not, then the substance is polar.
Polar substances have dipole moments.
Polar substances, so substances with ions or substances with dipole molecules, mix well with other polar substances; for example: water with alcohol.
Non polar substances mix well with non polar substances; for example: fat with oil.
Polar substances do not mix well with non polar substances (and v.v.): water and oil.
Short:
"ALIKES DO LIKE EACH OTHER".
From the above text must be clear that the presence of charges in a molecule has enormous influence on the molecule.
If in one molecule or in a complex ion two equal charges are close toghether, they will influence each other: repulsion;
that's how a substance gets special properties.
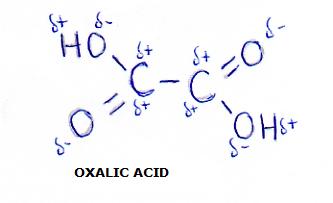
an example is ethane diacid, or: oxalic acid:
The C - C bond in the middle is under great tension (because of the repelling action of the positive charges).
That will weaken that C - C bond.
The consequence is that oxalic acid (different from the normal carbon acids) breaks easily (is easily to oxydise).
Normally a C-C bond is difficult to break.
Another example is: cationic acids (see module 9)
If you dissolve an Iron(III)salt in water, you get in the centre of a complex a small and strong positive Iron ion with close around it an number of water molecules.
The δ--side of the Oxygen atoms is attracted by that positive Iron ion.
The δ+-atoms of Hydrogen in the water molecules will become under the influence of that same Iron ion with a certain repulsion as a consequence.
Some complex ions can, because of this effect, even donate some H+ ionsand thus act as a weak acid (cation acid).