Optimal functioning and denaturating of enzymes
Proteins, and certainly the enzyms, are very sensitive for the environment in which they are, in particular the pH and the temperature. They have big influence on the threedimensional structure.
Denaturation for example, is desastrous: the enzyme (a protein) loses its tertiary structure and with that, its shape. That enzyme doesn't work any more.
So, deviation of the optimal pH and temperature will cause immediately some influence on the activity of the enzym.
A couple of possibilities to change the environment:
- Heating gives more movement to the atoms, on particles in general and on bondings.
The increased movement can disturb the vanderWaals and polar attraction.
The covalent bondings do not brake easily at heating.
If the denaturation was caused by temperature increase, that change is irreversible. Such an enzyme can be thrown away.
-
Adding acid or alcohol can brake the Hydrogen bridges.
Than the tertiary structure, and maybe the secondary structure can be lost.
Its also possible to change the pH or to dilute or to add alcohol or salts.
Such denaturation may be (partly) undone; there may be some reversibility.
In both cases, the tertiary and maybe the secondary structure can be lost.
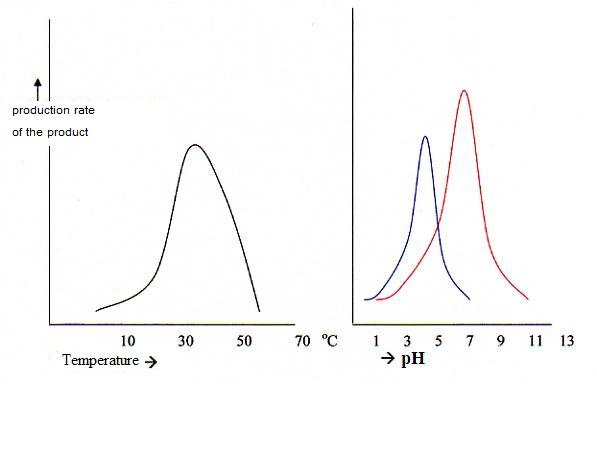
The figures show the sensibility of the enzyme activity for temperature and pH. (the enzyme activity is found op the y-ax)
High temperatures are desastrous for enzyms. This cooking is irreversible denaturation, unrecoverable.
Cautious and modest decrease of temperature only will decrease the enzyme activity, without any denaturation.
From that lower temperature going back to the normal temperature of 30 - 40°C, the activity will recover.
(just an idea: just before dying you freeze your body, hoping that in a hundred years it can be recovered)
The big part of the enzyms functions the best at pH values of about 7 (so neutral). But there are a couple of exceptions like pepsine. That one has an optimal pH value of 1 - 2.
Strong changes in pH can also denaturate an enzym. This can be reversible, but is not always.

