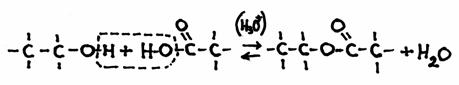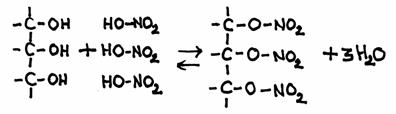Condensation
Two molecules connect and form one new molecule, with water as a side product.
molecule 1 + molecule 2  new molecule + water
new molecule + water
Example: the formation of an ester:
ethanol + ethanoic acid  ethyl ethanate + H2O
ethyl ethanate + H2O
in structural formulas
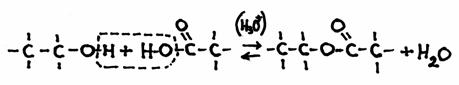
This is a reversible process, so the direct reaction is the condensation and the reverse reaction is called: hydrolysis.
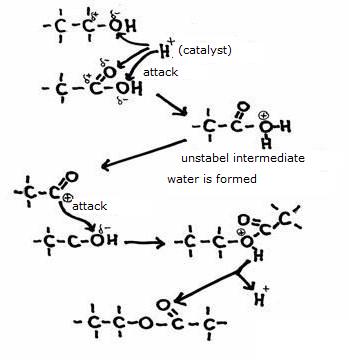
The production of an ester, from an alcohol and a carboxylic acid can be accelerated with a catalyst.
Therefor you can use, for example, an acid.
The H+-ions attack a negative position, and in that case we call the mechanism "electrophylic".
There are different mechanisms where negative particles attack positive positions of a molecule, and then we talk about 'nucleophylic'.
It happens that an alcohol reacts with a inorganis acid. For example, glycerol can react with nitric acid.
Normally glycerol reacts with fatty acids to produce fats and oils, but the reaction with nitric acid is comparable:
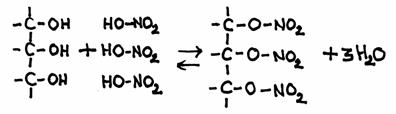
The product has an official name: glycerol-trinitrate.
But in practice you often hear the name: nitroglycerine
That name 'nitroglycerine' is not in accordance with to the IUPAC rules.
Another example of condensation (and of the opposite, hydrolysis) is the production of ether and water out of the reactants alcolhol + (another) alcohol,
for example:
Ethanol + propanol  ethoxy-propane + water.
ethoxy-propane + water.
This reaction needs a catalyst (can be an acid) as also higher temperatures.