Benzene and benzene derivates
How to approach the structure of benzene? How to look at it?
An easy way to do so is starting at the four valency electrons of the Carbon atom.
For not complicating, we leave out the orbitals and hybrides (spx).
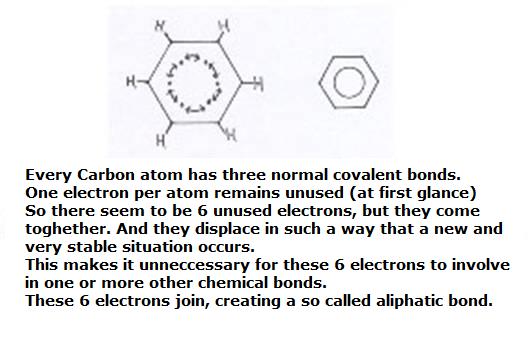
The benzene ring can be considered as built up of six Carbon atoms, every one of them using their valency electrons for bondings with an H atom and two neighbouring C atoms.
You see, then there is of each C atom still one valency electron unused; is still free.
What to do with these 6 free electrons? They toghether form a kind of ring bonding; a ring bond of 6 common C atoms.
Here we meet a special bonding that we indicate in structures with a circle.
Thus there is a similar bonding between all 6 C atoms of the ring.
Okay, it is a bit of easy explanation.
In the better chemistry books you can find lots of more explanation, more precize, with orbirals 2s, 2px, 2py
and 2pz.
Those three: 2s and 2px and 2py can mix, and form three new 'hybride-orbitals': sp2.
They are responsible for the formation of two bondings (we talk in term of orbital overlap) with other Carbon atoms and one bonding with a Hydrogen atom of the type σ.
Between every two C atoms there comes an overlap (bond) of 2pz-orbitals.
Or: 6 bonds of the type π.
For nomenclature of benzene derivates we chose one of the six C atoms in the ring. That one we call number 1.
Starting from number 1, we count numbers 2 - 6
In the old days (and still in many books) we used prefixes where now we use the numbers 1 - 6.
An example is: 1,2 dinitrobenzene was mentioned (and still you meet this name): ortho-dinitrobenzene (ortho = 1,2 )
- Ortho = 1,2
- Meta = 1,3
- Para = 1,4
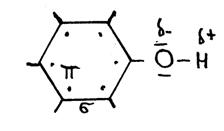
The above structure (C6H5OH) is the structureof "phenol".
The following needs your attention in nomenclature: phenol and phenyl; be carefull; they are not the same!
Phenol has an OH group at the benzene ring. But if benzene itself is a branch, than we call it 'phenyl'.


