Benzene
The molecular formula of benzene is: C6H6
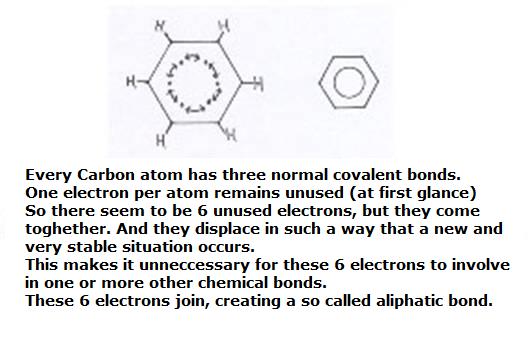
Every Carbon atom in the ring has three normal covalent bonds.
That's how every C-atom remains with a 'non paired electron' that still can connect with whatever other atom.
So in total six electrons are in stock, and these six now will connect in a very special way with each other, without other atoms.
A special π-bonding (a "pi-bonding) is made. We come tot that later.
Here we do not treat the very complicated bonding types, so you may simpify here by saying:
Those six electrons obtain the opportunity to dislocate all over the ring of the 6 atoms.
They are allowed to move freely over this ring, obtaing this way bigger freedom, more movability.
That means at the same time: that's how that ring becomes much more stable.
If molecules (in carbonchemistry) have this kind of ring structures with dislocation of electrons, we speak of aromatic molecules.
